Sections
- Introduction
- Objective: Zero Discharge
- Characteristics of Fortilife Membranes
- Practical Example
- Summary
INTRODUCTION
During the second half of the last century, reverse osmosis became established as a technique for desalinating water, both for drinking and industrial uses:
Initially, reverse osmosis membranes were very sensitive to fouling and would hydrolyze (cellulose acetate membranes). Later, with polyamide membranes, significant progress was made by operating at lower pressures and achieving better water quality. However, even so, the obtainable conversions hardly exceeded 75% in a single step, and working in two steps made it risky to exceed 85% due to polarization and salt precipitation issues.
Since then, research has focused on factors such as polarity, materials, types and arrangements of spacers, and the tails used as adhesives, resulting in membranes resistant to fouling, operable at high temperatures, and at extreme pH values and moderate concentrations of oxidants.
Recently, several types of membranes have been developed that, in addition to being more resistant to pH changes and having greater fouling resistance, can operate at very high pressures, allowing for operation with very high saline concentrations.
Factors such as the ionic strength of the solutions and the need for residence time for the generation of precipitation and crystallization germs have allowed permeate yields to exceed 95%, depending on the salinity and type of water being treated.
OBJECTIVE: ZERO DISCHARGE
The rejects from reverse osmosis plants have always represented a problem due to their high salinity, making it difficult to discharge them. Only points close to the sea have this possible destination for discharge, and within a demanding regulatory framework.
Thus, the best option from an environmental perspective is zero discharge. However, although the volume of effluent could be reduced to 15% of the raw water (85% conversion), the resulting flow remained too high to consider an evaporative process due to its significant installation and energy costs; moreover, in many cases, multiple evaporation stages are required to reach concentrations that allow the concentrate to be considered an exportable waste to a landfill (Concentration > 30%).
The search for the best available technologies has led to the development of reverse osmosis membranes (e.g., Fortilife membranes), making the approach to the ideal zero discharge significantly more viable, resulting in a concentrate flow of about 5% of the input.
With a significantly higher concentration factor (Fc > 20). Under these conditions, both technically and economically, the systems for reducing volume and increasing the concentration of residual effluents to the point of making them solid become more feasible, which is achieved with low-energy vacuum evaporation techniques, in which Condorchem Envitech has proven experience.
CHARACTERISTICS OF FORTILIFE MEMBRANES
Efforts are being made to diversify the types of reverse osmosis membranes used, depending on the application they are intended for. Below are the basic characteristics of Fortilife-type membranes:
CR 100 Membranes
This type of membrane is designed for water with organic residues and relatively high suspended solids content, such as tertiary treatments of wastewater treatment plants or for surface waters (rivers, swamps, lakes) with a high SDI due to their colloidal content and suspended organic loads.
These membranes foul less than conventional ones (approx. 50%), so their cleaning frequency is also lower, and they recover better with cleanings, as observed in the following tables:
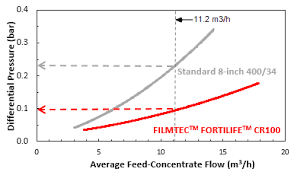
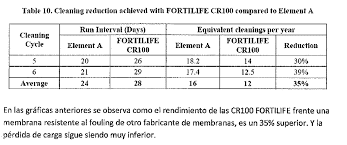
CR 100 membranes are not designed to work with high salinities (TDS < 15 g/l), as they cannot withstand more than 41 bar of pressure, and they have good salt rejection, making them ideal for use in the first step of the treatment line. XC 70 / XC 80 Membranes
These membranes, in addition to being resistant to fouling and having a high salt rejection, can withstand pressures of up to 83 bar, allowing them to work with high salinities (< 80 g/l) and are suitable for being arranged in a second or third step of the membrane set. XC-N Membranes
These are selective type membranes that allow operation up to 41 bar of pressure, with a salt rejection of 99%. They exhibit low fouling and low energy costs.
They can be considered as a high-pressure nanofiltration membrane with the advantages of the Fortilife type.
UHP Membranes
Finally, Ultra High Pressure (UHP) membranes are used to work in the final concentration stage, reaching pressures of up to 120 bar and very high concentrations (approx. 120 g/l).
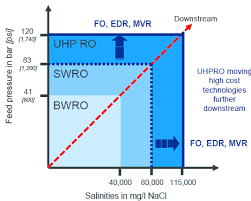
The following table summarizes the basic characteristics of each type of membrane for comparison.
Fortilife Membranes Table
| Product | Characteristics | Specifications | Advantages |
| CR 100 | High resistance to biofouling.
High salt rejection. Low salinity (< 15 g/l). |
Permeate flow = 44 m3/d.
Salt rejection = 99.7% Max input pressure = 41 bar |
Reduces cleaning frequency.
Biofouling < 50%. |
| XC 70 | Fouling resistance.
High salinity (15 – 70 g/l) |
Permeate flow = 30.6 m3/d.
Salt rejection = 99.7% Max input pressure = 83 bar |
Reduces cleaning frequency.
Longer operating time. Longer lifespan of elements. |
| XC 80 | Fouling resistance.
Low energy consumption. High salinity (15 – 80 g/l) |
Permeate flow = 34.2 m3/d.
Salt rejection = 99.4% Max input pressure = 83 bar |
Reduces energy costs.
Increases conversion. Reduces cleaning frequency. |
| XC-N | Selective ion separation.
Recirculation of purified concentrate. |
Permeate flow = 34.1 m3/d.
Salt rejection = 99%. Max input pressure = 41 bar. |
Makes rejection reusable.
Reduces fouling. Low energy cost. |
| UHP | Maximum salt concentration per membrane | Permeate flow = 28 m3/d.
Salt rejection = 99.7% Max input pressure = 120 bar |
Applicable for treating very high salinity waters, to achieve zero discharge |
To achieve an optimized design, the combination of these membranes in different stages is proposed, with the aim of simplifying the complexity of the installation and minimizing the size of the final evaporation installation.
For example, if we have a conventional two-step reverse osmosis installation and achieve an 85% conversion, the concentration factor will be FC = 1/(1-0.85) = 6.7.
If we start with water with a TDS of 2 g/l, the concentrate would have a TDS of approximately 13.4 g/l; if the input flow is, for example, 100 m3/h, we would need to design an evaporator for a flow of 15 m3/h with a TDS of 13.4 g/l.
To reach a concentration of 300 g/l in the residue, it will need to be evaporated in several stages, with the investment and energy costs that this entails.
Now suppose we have low-pressure, high-performance membranes (CR 100) in the first step, then we have XC70/XC80 membranes in a second step to work at high pressure and conversion, and finally, we have ultra-high pressure (UHP) membranes, after passing through a purification step in the reject using other XC-N type membranes.
In this case, we can achieve a salt concentration of approximately 100 to 150 g/l at a flow rate of 1.5 – 2 m3/h, making the vacuum evaporation process much more viable.
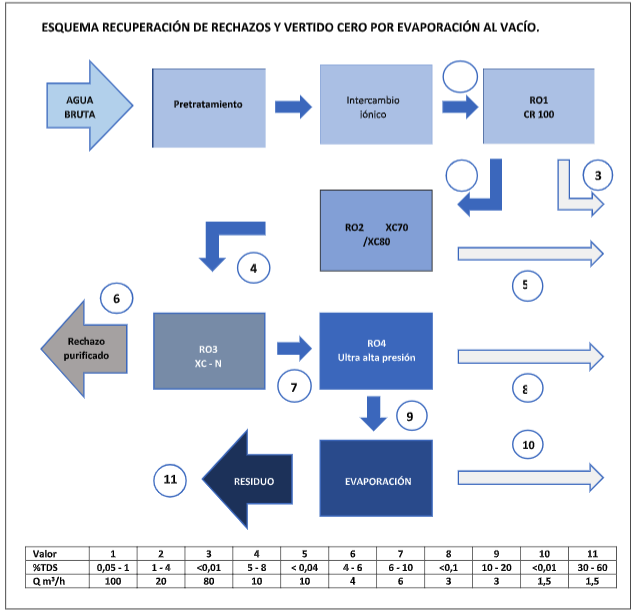
PRACTICAL EXAMPLE
We start from the reject of a reverse osmosis plant that takes a raw water flow of 830 m3/h from a swamp, with a TDS of 1,160 mg/l.
We can use CR100 membranes, which, due to their low fouling and good salt rejection performance, will simplify the ion exchange installation that will need to be used to treat the permeate and thus achieve the required salinity levels in the application of this example (demineralized water for the energy sector).
Moreover, the specification requires obtaining a flow of 120 m3/h of service water that must have a TDS <100 mg/l, and achieving zero discharge is important.
In the following projection, we start from the reject obtained from the reverse osmosis plant with CR 100 type membranes, and we observe how the membranes are combined to optimize the set, using booster pumping systems, counter pressures in the permeate, and recirculations.
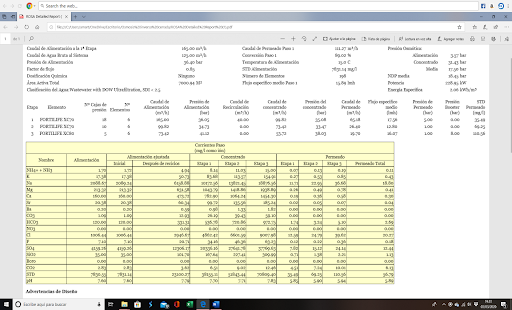
In this case, the achieved conversion is 89% and the FC = 9. The final reject flow would be 13.7 m3/h and its TDS = 70.8 g/l.
If we calculate the rejection percentage over the raw water flow, we have = 13.7/830 = 1.65%, then the system’s conversion would be approximately 97 – 98%, considering that water is consumed in pretreatment, washings, etc.
The TDS of the permeate is 53 mg/l and the specific energy consumption is 2 kW/m3, thus achieving the desired quality for service water, with an adequate energy consumption.
If we take the reject from this step and subject it again to concentration using specific membranes like the UHP, we achieve a conversion of 50%, which translates to a permeate flow of about 6 m3/h and a salinity of 83 mg/l, while the reject will have an equivalent flow and a concentration of 140 g/l.
It would be advisable to have two parallel reverse osmosis lines in this step to avoid salt crystallization. In this last step, it is recommended to work in a discontinuous regime, and each time the installation stops, the membranes should be rinsed and the effluent recirculated to the head of the installation.
We see that the vacuum evaporation solution would now be more suitable, both technically and economically, as it is very likely that the desired concentration level required in the residues of 30% will be reached, with a reasonable investment and operating cost.
In the example at hand, it is very likely that there is a return of condensates from your installations, which can be used to provide energy through a heat exchanger; in this case, a suitable equipment could be from the Envidest MVR FF series, followed by a crystallizer.
The permeate and condensate flow obtained will be very close to the 120 m3/h required for service water, and the salinity will be below 100 mg/l.
SUMMARY
Technological evolution in water treatment is allowing us to achieve the goal of minimal emissions to the environment. Many of the effluents that were discarded until recently, or whose treatment had an unfeasible cost, are becoming more accessible, while a large part of the captured water is reused and energy consumption is reduced.
This article highlights the significant step that has been taken regarding desalination by reverse osmosis with its latest generation of membranes and its possible symbiosis with the variety and specialty of evaporative treatments that can be combined to achieve the best result.
Bibliography and Internet Consultations.
https://www.dupont.com/brands/filmtec-fortilife.html
http://www.catedradelagua.uji.es/webcta/wp-content/uploads/2018/02/13_Ponencia_SGallego.pdf
“`