NOx reduction or removal
DeNOx® Recovery is a process for the treatment and reduction of NOx emissions.
The DeNOx® solution is based on the utilization of Selective Catalytic Reduction (SCR), which is one of the most competitive and efficient technologies for removing NOx from a gas stream. In our process, Selective Catalytic Reduction is combined with other processes such as photooxidation, gas scrubbing using scrubbers, or crystallization, to ensure maximum process efficiency in every situation.
The main advantages are:
- 99% reduction of NOx.
- Recovery of salts.
- Elimination of chemical waste.
- No harmful compounds released into the atmosphere.
DeNOx® is a NOx emission reduction system that can be installed in its entirety or partially, depending on the specific characteristics of each application. The detailed design of the process does not impose any limits on the application of this innovative and efficient treatment in situations where the efficient and competitive removal of NOx is required, such as power plants, some industrial facilities, cement plants, waste incineration plants, glass manufacturing plants, or refineries.
Our technologies for NOx removal
Advantages of DeNOx® Recovery
The main advantages of the DeNOx® process over conventional alternatives are:
- NOx removal yields of over 99%
- Conversion of waste into reusable raw material in the solar thermal energy accumulation process
- No chemical waste produced
- No hazardous compounds in atmospheric emissions
- Copying the natural atmospheric self-regeneration mechanism
- Simple and reliable operation
- Fully automated and robust operating process
Process characteristics
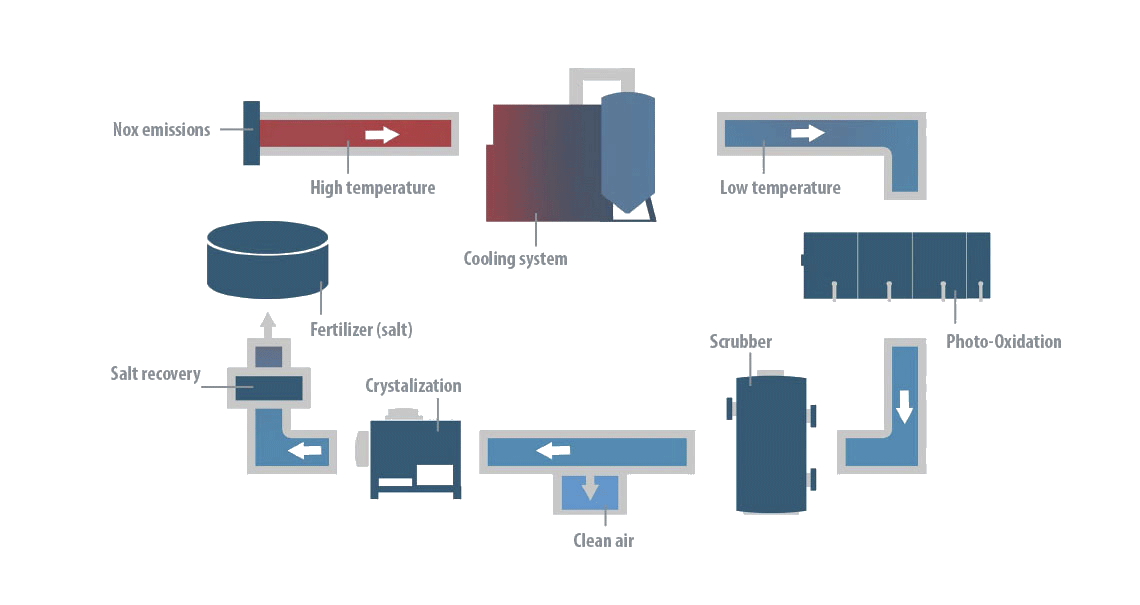
The DeNOx® process emulates the natural, self-purification mechanisms of the atmosphere itself, through a controlled combination of NOx emissions with water vapor, ozone and ultraviolet radiation.
The process is based on 4 stages:
- Photooxidation of the gaseous stream, producing ozone (O3) and hydroxyl radicals (OH·):
O3 + H2O → 2 OH· + O2
- Conversion of the nitrogen oxides (NO and NO2) into nitric acid (HNO3) through the high reactivity of the ozone and hydroxyl radicals:
NO + O3 → NO2 + O2
NO2 + OH· → HNO3 - Absorption of nitric acid in a gas scrubbing process and its neutralization by an alkaline solution of NaOH or KOH, obtaining the respective salts (NaNO3 or KNO3).
- Recovery of the salts formed in a crystallization process and subsequent re-use in the energy storage system itself.
This elegantly simple process guarantees that the NOx treatment is highly effective, combining high efficiency and robust and reliable operation.
NOx Reduction in solar thermal power plants
The DeNOx® system is the highest-performing process for effectively removing the NOx generated in the energy storage system of a solar thermal power plant. This energy storage system is based on the heating of salts, which are a mixture of sodium nitrate and potassium nitrate.
The DeNOx® process, patented by Condorchem Envitech, has been specially designed for treating emissions generated in solar thermal power plants, converting the pollutants (NOx) into products that can be reused in the solar thermal plant process.
In a solar thermal power plant, sunlight is concentrated using mirrors onto a receiver that reaches temperatures of up to 1,000°C. This heat is used to heat a fluid and generate steam, which drives a turbine and produces electricity. While early plants could only operate during sunlight hours, it is now possible to store the heat for nighttime production.
The energy obtained from solar irradiation is stored in a mixture of sodium nitrate and potassium nitrate, known as salts, which have a suitable melting point. They are in a liquid state, which requires a temperature of 280°C. The liquid salts are heated until they reach a temperature of 565°C, at which they are stored.
The innovation lies in the fact that electricity generation depends on the level of stored hot salts rather than solar radiation. To produce electricity, the hot salts are used to boil water in a heat exchanger, generating steam at 540°C and 100 bars. The steam, through a turbine, produces electricity based on real-time demand.
Despite the numerous advantages of this technique, it also poses a significant environmental problem. The liquid salts release nitrogen oxides (NOx) as discontinuous emissions with variable concentrations during the heating process.
What are NOx?
Nitrogen oxides (NOx) are a group of chemical compounds that contain nitrogen and oxygen atoms. They are toxic gases primarily generated as byproducts of combustion reactions in internal combustion engines, industrial processes, and the burning of fossil fuels.
The term NOx refers to all gaseous chemical compounds formed by nitrogen and oxygen, although it primarily refers to nitric oxide (NO) and nitrogen dioxide (NO2). NO is a colorless and odorless gas, while NO2 has a brown color and a strong, unpleasant odor. Both gases are known to be highly detrimental to the environment and human health.
The World Health Organization (WHO), in its Air Quality Guidelines, states that nitrogen dioxide, even in short-term exposures at high concentrations, can have severe health effects (respiratory and ocular system irritation, development of chronic respiratory and cerebrovascular diseases, among others). Additionally, NOx can contribute to the formation of secondary pollutants once they are emitted into the atmosphere.
NOx emissions must be appropriately treated to release gas streams into the atmosphere without causing any environmental impact.