Sections
- Management of effluents in pharmaceutical production
- Production of wastewater in the pharmaceutical industry
- Treatment of discharges with emerging contaminants
- Advanced oxidation processes (AOP) + Biological treatments
- Vacuum evaporation
- Advanced oxidation (AOP) + Evaporation
- Vacuum evaporators for the recovery of active pharmaceutical ingredients (API) in wastewater
- Summary
Management of effluents in pharmaceutical production
Every year, more than 100,000 tons of pharmaceutical products are consumed worldwide. Active pharmaceutical ingredients (API) are a source of environmental contamination, both during their manufacture and later during their use and disposal.
The wastewater generated in this sector is highly variable in terms of flow and composition, making treatment complex.
There are various technologies for the biological treatment of these discharges, among which are: Biological treatment by activated sludge, MBBR biological process (fixed biomass on a moving bed), anaerobic treatments, selective micro contaminant treatments, etc.
The problem is that in many cases, issues arise due to inhibitory and toxic contaminants, as well as by organic compounds resistant to oxidation.
For this reason, many companies in the pharmaceutical industry entrust the management of their effluents to specialized waste managers, incurring high costs for the disposal of their liquid waste.
In this scenario, vacuum evaporators stand out for their high efficiency and economic profitability when treating the wastewater produced by the pharmaceutical industry.
Production of wastewater in the pharmaceutical industry
In the pharmaceutical sector, purified water is used for the preparation of specific products and, on the other hand, water is also used for the sanitation of equipment, containers, and primary packaging. As a result, effluents are obtained that are characterized by containing residues of chemical products, such as leftovers from drugs and/or detergents used in cleaning.
The World Health Organization indicates that a wide variety of waste generated by the pharmaceutical sector mixes with environmental water through the effluents from manufacturing or production facilities.
The volume and composition of wastewater from this industry show great variability due to the different modalities in production processes and the composition of the drugs.
In the processes of manufacturing pharmaceutical products, substances containing chemical products, cleaning products, and the so-called APIs, or active ingredients, which are the basis of medicines marketed for the prevention and treatment of diseases, are used.
These abnormal contaminants for the environment are called “emerging contaminants” (EC), and they are compounds that are discharged into water and are not regulated. Emerging contaminants, also called micro contaminants, are chemical compounds resulting from human activities carried out in daily life, such as personal hygiene or health care. They are substances of different origins and chemical compositions, of which relatively little is known regarding their impact on the environment and on humans.
Treatment of discharges with emerging contaminants
In general, biological treatment processes are the most economical and common for treating wastewater, but the complexity of the components present in the wastewater from the pharmaceutical industry complicates the application of a specific treatment. Likewise, the presence of certain compounds such as antibiotics and disinfectants prevents the complete removal of contaminants through conventional biological treatments.
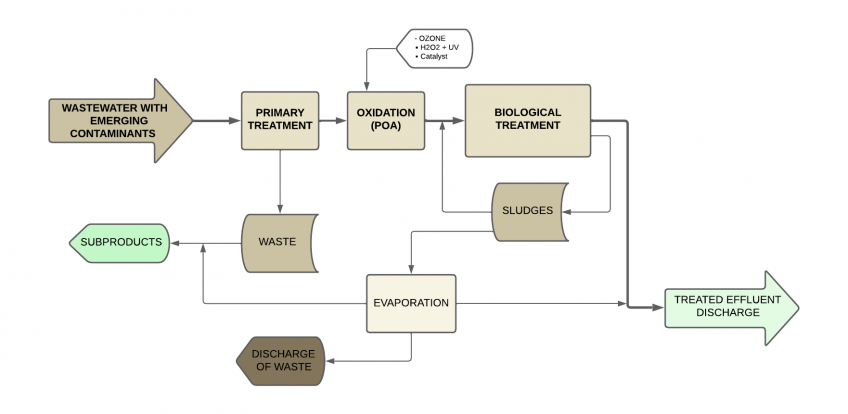
Advanced oxidation processes (AOP) + Biological treatments
Advanced oxidation processes (AOP) have proven to be highly effective for wastewater produced in the pharmaceutical industry, especially those containing micro contaminants or emerging contaminants.
This technology uses chemical oxidants to reduce chemical oxygen demand (COD) levels, as well as to break the bonds of these organic contaminant compounds and facilitate their biodegradability, thus saving reagents and reducing operational costs compared to attempting to oxidize all organic matter with reagents.
These treatments are based on physicochemical processes capable of producing profound changes in the chemical structure of contaminants, involving the generation and use of powerful transient species, mainly the hydroxyl radical (OH-), which is the strongest oxidizing agent after fluorine. Additionally, the generation of radicals occurs from oxygen, hydrogen peroxide, and supported catalysts, so the reaction byproducts are only water and carbon dioxide.
Among the main technologies of advanced oxidation processes, the Fenton process and its variants stand out, which consist of the addition of iron salts that act as catalysts, in the presence of hydrogen peroxide (H2O2), in an acidic medium, and under certain pressure and temperature conditions, to form OH- radicals.
After the AOP process, biological treatment can continue, which in many cases will allow compliance with discharge limits and even reuse the treated water in the industry’s own services or for irrigation.
Vacuum evaporation
Vacuum evaporation is a process that involves the removal of water and other solvents present in a liquid waste by applying heat under vacuum conditions.
By reducing the atmospheric pressure on the solution, the boiling point of the liquid is lowered, allowing it to evaporate at lower temperatures.
Contributions of the process
Vacuum evaporation is a technology widely used by the pharmaceutical industry. Below, we summarize how this process contributes to the management of wastewater generated in this industry:
- Concentration of contaminants and minimization of residual effluent: During evaporation, the water is heated and turns into vapor. The organic and inorganic contaminants present in the water do not evaporate and remain concentrated in the residual solution. This allows for a reduction in the total volume of wastewater that must be treated later and separates a significant portion of the contaminants.
- Recovery of valuable products and reuse of water: The concentrated products can be valuable for the industries that generate them, such as APIs for pharmaceutical industries. Their recovery requires a controlled evaporation process under specific operating conditions to prevent deterioration or loss of qualities.
- Elimination of micro contaminants: Although evaporation does not completely eliminate micro contaminants, it can concentrate them. Subsequently, additional techniques (such as adsorption or filtration) can be applied to remove these concentrated contaminants.
- Energy efficiency: Vacuum evaporators have improved their operational efficiency to reduce energy consumption, such as evaporators with heat pumps or those that work with steam thermocompression.
Limitations of the process
Evaporation is a valuable technique in the treatment of wastewater from the pharmaceutical industry, but it also has its limitations. Below, we will explore some of them:
- Complexity of effluents: Pharmaceutical wastewater can contain a variety of compounds, including active pharmaceutical ingredients (API), chemicals, and micro contaminants. Evaporation may not always efficiently remove all these components due to their complexity.
- Concentration of contaminants: While evaporation concentrates contaminants in the reject (the non-evaporated fraction) and minimizes the volume of waste to be sent to a manager, this reject of concentrated compounds can still be harmful to the environment and must be managed properly.
- Limitations in the removal of micro contaminants: Although evaporation can concentrate micro contaminants, it does not always eliminate them completely. Additional steps may be required to address these specific compounds.
- Need for complementary technologies: To address the limitations of evaporation, it is often combined with other treatment techniques, such as adsorption and biodegradation. The choice of the appropriate technology depends on the specific composition of the wastewater.
Advanced oxidation (AOP) + Evaporation
The combination of advanced oxidation and vacuum evaporation is a high-performance solution for treating wastewater generated by the pharmaceutical industry.
Advanced Oxidation Processes (AOP) are methods that use highly reactive hydroxyl radicals (OH-) to oxidize and degrade organic contaminants. These processes have advantages such as the ability to mineralize organic compounds and reactivity with a wide range of contaminants.
However, advanced oxidation processes also have disadvantages, such as the cost of reagents and energy requirements. Therefore, they are often combined with other treatments.
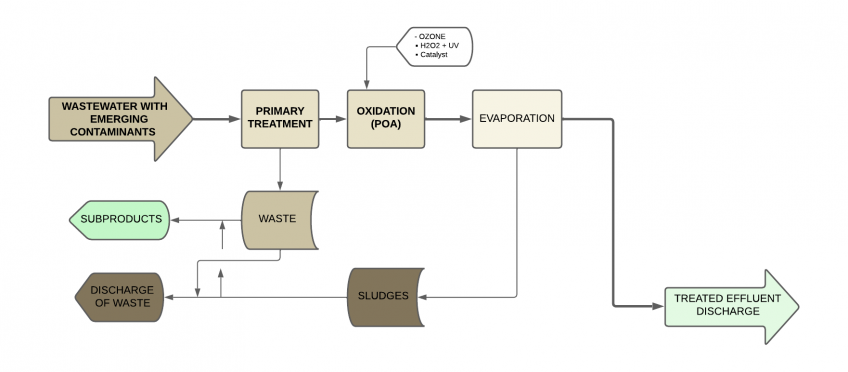
As we have discussed, vacuum evaporation is a technique that concentrates contaminants by converting water into vapor. It can reduce the total volume of wastewater and allow for the recovery of valuable products. However, evaporation also has energy costs and does not completely eliminate micro contaminants.
The combination of AOP and evaporation offers high efficiency, as AOP can degrade difficult-to-treat organic compounds, and evaporation can concentrate contaminants before applying other treatments. This combination of processes is especially suitable for low flows and high contaminant loads.
Vacuum evaporators for the recovery of active pharmaceutical ingredients in wastewater
APIs are chemical substances that must be handled with extreme caution due to their potential impact on public health. Inadequate treatment of APIs can lead to issues such as:
- Cross-contamination
- Loss of drug potency, or
- Appearance of impurities, which could jeopardize patient health and compromise the reputation of the pharmaceutical company.
The production processes of APIs follow the following basic outline:
- Reception and storage: APIs usually arrive at the pharmaceutical company’s facilities in the form of powders, granules, or liquids. It is crucial to have rigorous procedures for the reception and storage of these materials, ensuring their correct identification, integrity, and traceability.
- Handling and processing: During the manufacture of medicines, APIs are handled and processed to formulate the final products. This process includes operations such as mixing, granulation, compression, and encapsulation, which must be carried out following strict good manufacturing practices (GMP) protocols.
- Analysis and quality control: Thorough analyses are conducted to verify the identity, purity, and potency of APIs, as well as to detect the presence of impurities. These quality controls are essential to ensure that medicines meet regulatory standards and are safe for use.
- Storage and distribution: Finished APIs must be stored under appropriate conditions to preserve their stability and avoid contamination. Additionally, safe procedures must be established for the distribution of APIs to other manufacturing facilities or to end customers.
The manufacture of APIs is subject to strict controls by regulatory agencies. It is crucial for companies to comply with these regulations and apply good manufacturing practices to ensure the quality and safety of pharmaceutical products.
These compounds, along with other chemical ingredients, are released into the environment through wastewater, creating a problem that needs to be addressed at the source, that is, during the production process, through the use of efficient technologies for managing these residual effluents.
In this regard, companies are increasingly aware of the need to follow a sustainable environmental policy and seek solutions that minimize not only the volume of waste produced but also improve the efficiency of its disposal.
Conventional wastewater treatments do not always succeed in eliminating all residues of active pharmaceutical ingredients, as they are very complex waste to treat.
Vacuum evaporators are the most efficient technology for separating, concentrating, and reusing APIs, as well as other contaminants present in the wastewater produced by the pharmaceutical industry.

Technological advances made in recent years have allowed for a reduction in the operating costs of vacuum evaporators, making them a technology that meets the need to treat complex effluents, such as those produced in the pharmaceutical sector.
The separation of active pharmaceutical ingredients (API) by evaporation is a method used in the pharmaceutical industry to purify or concentrate chemical substances. This process involves the controlled evaporation of the solvent in which the active ingredient is dissolved, leaving behind the compound to be recovered in solid or concentrated form.
Vacuum evaporators allow for the concentration and recovery of APIs used by the pharmaceutical industry, such as solvents or chemicals used in drug synthesis.
The evaporation process can be carried out in several ways:
- Vacuum evaporation
- Evaporation at room temperature
- Evaporation by heating.
The choice of method depends on the properties of the active ingredient and the solvent used, as well as the specific requirements of the process.
Vacuum evaporation is an effective technique for the separation and concentration of active ingredients, but it is important that the process is carried out under the appropriate conditions to avoid degradation or loss of purity of the compound of interest.
Therefore, factors such as:
- Temperature: The evaporation temperature varies depending on the active ingredient and the solvent used. In general, a temperature is selected that is high enough to promote efficient evaporation of the solvent but low enough to avoid degradation or volatilization of the active ingredient. Therefore, temperatures are usually in the range of ambient to moderately elevated, depending on the needs of the process and the stability of the compound.
- Pressure: The pressure can also vary depending on the specific conditions of the process. In many cases, reduced or vacuum pressure is used to facilitate evaporation at lower temperatures, which helps prevent thermal degradation of the active ingredient and the solvent. Reduced pressure lowers the boiling point of the solvent, making it easier to evaporate at lower temperatures.
- Exposure time: The time during which the active ingredient is kept under evaporation conditions is also important. A balance is sought between the efficient removal of the solvent and the minimization of the active ingredient’s exposure to conditions that may cause its degradation.
The evaporation of active pharmaceutical ingredients (API) can be carried out under different pressure and temperature conditions, depending on the physical and chemical properties of the active ingredient and the solvent, as well as the specific requirements of the process.
In summary, the evaporation of active pharmaceutical ingredients is typically carried out at moderate temperatures, with reduced or vacuum pressures to facilitate evaporation and minimize degradation, and with controlled exposure times to ensure the quality of the final product.
For the evaporation of active pharmaceutical ingredients (API) under vacuum, several types of evaporators can be used that can be adapted to the specific needs of the application:
- Falling film vacuum evaporators: These evaporators use a falling film design to maximize mass and heat transfer efficiency. They are suitable for concentrating viscous and heat-sensitive solutions, as they allow for a short residence time and gentle evaporation.
- Multiple effect evaporators: They allow for high concentrations in the products to be dehydrated due to their multiple effect operation.
- Heat pump evaporators: They allow for the evaporation of the discharge containing the product to be dehydrated at low temperature and under pressure conditions in a moderate exposure time. They are suitable for concentrating high-viscosity solutions and APIs prone to degradation at high temperatures.
- Vacuum crystallizers with heat pump: These devices achieve high concentration values of the product to be separated and dried, with low consumption and high yield, respecting the stability of the API compounds.
In general, the choice of the appropriate evaporator will depend on several factors, such as the properties of the active ingredient and the solvent, the sample volume, heat sensitivity, and the specific requirements of the process. It is important to select an evaporator that offers precise control of the evaporation temperature and pressure, as well as high mass transfer efficiency, to ensure efficient evaporation and high quality of the final product.
Conclusion
The manufacture of medicines and the cleaning of equipment generate wastewater with emerging contaminants (EC) that are difficult to remove with traditional treatments.
Among the various technologies available, vacuum evaporation presents itself as an efficient and cost-effective solution for treating discharges from the pharmaceutical industry, although it is important to consider its limitations and combine it with other strategies to achieve comprehensive and responsible management of these complex wastewater.
In a context of increasing environmental awareness and stricter regulations, vacuum evaporation is expected to remain an attractive option for the treatment of discharges in the pharmaceutical industry.
Bibliography and inquiries:
Wastewater treatment of the pharmaceutical industry through the ozone technique
Industrial wastewater treatment | Condorchem Enviro Solutions