Sections
- Advantages and disadvantages
- Classification of membranes
- By particle size
- By particle charge
- Membrane configuration
Advantages and disadvantages
Among the processes that have evolved the most in recent decades are membrane filtration. Generally, these consist of forcing the liquid to be filtered through a membrane placed on a solid support.
They work because certain types of membranes allow the passage of particles with specific characteristics while preventing the passage of those that do not possess the same characteristics.
The need for increasingly higher permeate flows, produced at lower operating pressures, has led to constant advancements in the design and manufacture of membranes.
Membrane separation operations are widely used, and their use surpasses conventional methods due to their ability to produce separations very efficiently at room temperature and their cost/efficiency ratio. Below are the main advantages and disadvantages in relation to other technologies:
Advantages
- They offer high separation efficiency where the key factor is the cut off of the membrane.
- They are processes that can be carried out at room temperature and continuously.
- Energy consumption is not high, and the use of chemical reagents is not required (except for anti-fouling agents to clean the membranes).
- The ease of combining this technique with other processes.
- Very compact plants that require little physical space.
Disadvantages
- It is not a technique that eliminates the contaminant but rather concentrates it.
- A reject/residue stream is generated that must be treated properly.
- The cost of membranes and their durability must also be considered. It is important to pre-treat the effluent to extend the lifespan of the membranes.
- Depending on the specific application, problems of degradation, fouling, or polarization of the membrane may arise. These problems, while solvable, complicate and increase operating costs.
Classification of Membranes
Currently, there are many different classes and types of membranes that allow the passage of certain solutes depending on their nature, ionic charge, or size.
In this article, we will focus on the classification of membrane processes according to the separation factor.
| SEPARATION FACTOR | DRIVING FORCE | TYPE – OPERATION |
| Size | Pressure | Filtration |
| Microfiltration | ||
| Ultrafiltration | ||
| Nanofiltration | ||
| Size / Diffusivity | Pressure / Concentration | Reverse Osmosis |
| Charge / Diffusivity | Electric field | Electrodialysis |
| Reversible Electrodialysis |
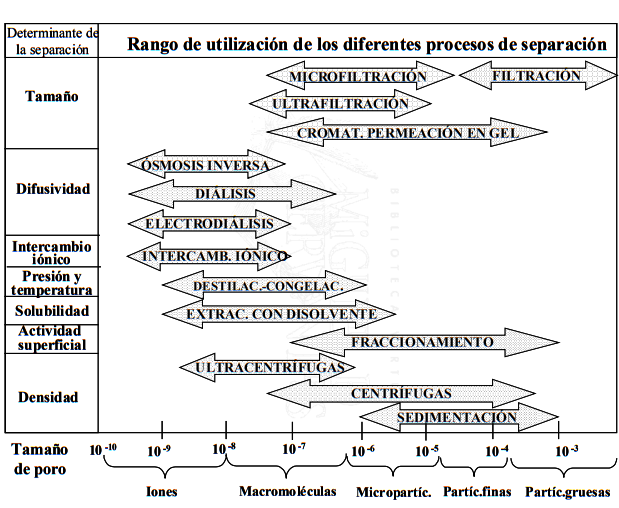
Source: Structural and surface characterization of microporous membranes, Laura Palacio Martínez, 1999 – University of Valladolid
By particle size
Depending on the size of the particles to be separated from the liquid, the type of membrane to be used will vary, with options including filtration, microfiltration, ultrafiltration, nanofiltration, and reverse osmosis.
In all these processes, the driving force is pressure. Below are the differences between them:
Filtration
Conventional filtration uses a porous medium made of granular material (gravel, sand, anthracite, etc.) as the filtering medium.
The liquid to be filtered passes through the porous bed, either by gravity or under pressure, with solids trapped in the interstitial spaces between the particles that make up the filtering bed.
The alternative to filtration using porous beds is the use of filters made from agglomerates of synthetic fibers such as polycarbonate or cellulose. Depending on the material used and its arrangement, the average pore diameter of the filter varies, which is the parameter that determines the minimum size of particles that will be retained (cut off or filter cut-off value).
These filters are folded inside a cartridge and can retain particles larger than 10 mm (sand particles, fine dust, etc.). They allow for flow densities of 4 to 8 m3/(m2·h), which, although the flow densities of granular filters are similar, the latter require much more physical space to provide the same filtration area.
However, granular filters can undergo backwashing, which is very effective. Thus, to filter an effluent with a high solid content, granular filters are the most convenient option. When the solid content is low or moderate, filtration cartridges are more competitive and require less space.
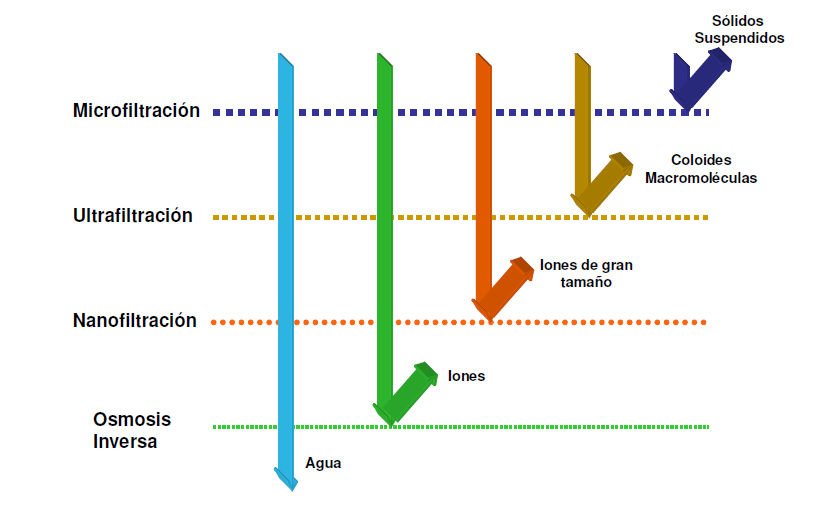
Microfiltration
Microfiltration membranes separate particles ranging from 0.1 mm to 10 mm (batteries, very fine coal dust, asbestos, etc.). These membranes can be made of nylon, polyethylene, polypropylene, etc.
Ultrafiltration
Ultrafiltration membranes retain particles ranging from 1 nm to 100 nm (0.1 mm), which is the size of viruses, colloids, macromolecules, endotoxins, etc.
The mode of operation is equivalent to that of microfiltration; the membrane set is placed on a support, and a pump increases the pressure of the liquid to pass through the membrane.
Nanofiltration
While microfiltration and ultrafiltration separate suspended particles from the liquid, nanofiltration can separate dissolved molecules in the liquid (sugars, proteins, dye molecules, etc.).
Nanofiltration membranes have a cut-off value between 0.1 nm and 1 nm, which is the typical size of most molecules that do not have a high molecular weight.
Even ions such as Ca2+ and Mg2+ are retained, making it possible to use these membranes to remove water hardness without dosing chemical reagents.
Reverse osmosis
Reverse osmosis is a phenomenon based on the equilibrium established on both sides of a semipermeable membrane that separates two volumes of liquid with different saline concentrations. The solvent diffuses through the membrane, while dissolved ions cannot.
Naturally, the solvent would pass from the more diluted saline solution to the more concentrated one to equalize osmotic pressure (osmosis). However, if pressure is applied on the side of the more concentrated solution, the flow through the membrane is reversed, resulting in a net flow of solvent crossing the membrane from the more concentrated solution to the less concentrated one. The pressure that must be applied depends on the concentration of salts in the concentrated solution.
In microfiltration, ultrafiltration, and nanofiltration, all fluid passes through the membrane while solids are retained on the membrane surface.
In the case of reverse osmosis, as the solution increases its salt concentration, the applied pressure must also be higher, and the flow is tangential to the membrane. In this way, part of the solvent crosses the membrane, and the other part drags all the dissolved salts, molecules, and particles contained in the feed.
Thus, there is a feed flow and two effluents, the permeate and the reject, where all the dissolved salts, molecules, and particles contained in the feed are concentrated.
Depending on the type of membrane used, the operating pressure, and the characteristics of the effluent to be treated, the proportion between the permeate flow and the feed flow varies, ranging from 50% to 75%.
To extend the life of reverse osmosis and nanofiltration membranes, it is advisable to pre-treat the effluent, usually through ultrafiltration.
Numerous industrial sectors use reverse osmosis to produce high-purity water, such as the pharmaceutical industry, the food industry, nuclear power plants, the electronics industry, the biotechnology industry, etc.
In environmental applications, reverse osmosis is also used to reduce and/or maximize the concentration of residual effluents, a process generally followed by a vacuum evaporation-concentration stage to fully concentrate the residue. Reverse osmosis is also used to refine condensed water in evaporation processes where residues are concentrated.
As a standard result, reverse osmosis returns 80% of purified water and a 20% reject.
By particle charge
Electrodialysis
It consists of the removal of electrically charged ions that are dissolved in water. To carry out this removal, a pair of electrodes with different electric charges is introduced into the feed water so that the dissolved ions will be attracted to the electrodes of the opposite sign.
This procedure allows for the displacement of ions from one place to another in the solution.
The use of selective anionic and cationic membranes alternately is essential so that the feed water loses negative and positive ions after passing through the separation zone.
It is interesting to place the membranes alternately so that solutes concentrate in some channels, in a water referred to as concentrated, while in other channels, the feed water circulates, gradually losing its contaminants until it exits the process with a very low salt concentration.
Reversible Electrodialysis
In this case, the polarities of the electrodes are periodically altered so that the water flows temporarily change direction, allowing the previously concentrated channels to receive purified water and vice versa.
This method eliminates the risk of precipitate formation, fouling, and membrane blockage, as the periodic change in the direction of water flow helps clean conduits and membranes, in addition to preventing the appearance of slimes and other deposits in the plant.
Membrane Configuration
There are commercial equipment with different arrangements of membranes to adapt to different conditions.
Thus, we can find the following configurations:
Membrane cartridge
The membranes are folded around the permeate collector. They are compact systems, ideal for treating solutions with a low concentration of suspended solids and are usually used with filtration and microfiltration membranes.
Spiral membranes
A set of membrane sheets, separated by a porous support, is wound around a tube that acts as a permeate collector. It is a very compact design, offers a good cost-efficiency ratio, and is suitable for large volume applications.
It is generally used with nanofiltration and reverse osmosis membranes.
Tubular membrane
Tubular membranes are placed inside a rigid housing. The feed enters through the inside of the membranes, and the flow is directed outward. Due to the diameter of the membrane tube, which is 5 to 10 mm, it is unlikely that there will be clogging issues. It is suitable for effluents with a high concentration of suspended solids. It is usually used for ultrafiltration applications.
Plate and frame filter
It physically resembles a filter press. The membranes are placed on frames separated by plates, and the feed flows through the space between the plates and the membranes. Solids concentrate on one side of the membrane, and the permeate is evacuated on the other side.
This arrangement is only used when the feed has a high viscosity, generally in applications in the pharmaceutical and food industries.
Hollow fiber
It consists of a large number of membranes with a diameter of less than 0.1 mm that form a bundle inside a housing.
It is practically used only for nanofiltration and reverse osmosis applications to treat effluents with a low concentration of solids.