Sections
- Presence of contaminants in wastewater
- Separation processes for wastewater
- Concentration of separated contaminants
- Summary
Presence of contaminants in wastewater
The purest natural water is found in the atmosphere in the form of vapor. During its precipitation on land as rain, it carries with it impurities that are suspended in the air (dust, pollen, etc.). A significant portion of this water ends up in the sea, which helps maintain the saline balance and purification of this medium, while the rest contributes to the solid surface of the planet, nourishing rivers, swamps, and lakes, as well as underground aquifers. Additionally, we have the water that precipitates in solid form during winter periods and/or at high points in the mountains.
Pure water tends to combine in search of stability, making it a good solvent. Thus, the soluble mineral salts it encounters along the way integrate into its body through a process known as dissolution, which significantly contributes to the phenomenon of erosion and transformation of the land.
These dissolved salts contribute to the potability and conductivity of water, but when certain salinity limits are exceeded, they become a contaminant that needs to be managed.
The removal of these salts is also carried out when process water with low or no salinity (demineralized water) is required, or when it is necessary to selectively separate certain specific contaminants, as in the case of softening, removal of nitrates, or selective separations of certain cations and anions.
In addition to inorganic compounds, water can also contain organic contamination of plant and animal origin, as well as other contaminants such as organic compounds, bacteria, viruses, etc. However, a large part of the contamination of water comes from human activity, both domestic and industrial.
Water purification technologies allow for the separation of different types of particles based on their size and nature. In this way, water is regenerated for reuse or discharge.
These technologies can be grouped based on the magnitude on which they rely to carry out the separation.
Separation processes for wastewater
Separation of contaminants by mass
For the separation of large or medium-sized solids (up to approximately 1 mm), purely physical systems are used. These include screens, sieves, and grids, which are nothing more than open filtering processes with a passage size suitable for the size of the solids. The extraction of the separated solids is mechanical and is usually done using manual rakes or automatic combs.

Other methods of physical separation include decantation and flotation, which allow for the removal of suspended materials. Flotation combines sedimentation with the flotation of less dense materials, with or without the aid of blown air.
Decantation and centrifugation are separation processes that take advantage of the difference in mass and density of the particles in relation to the solvent to achieve separation. The heavier the particles, the easier their separation.
Chemical reagents (coagulants and flocculants) are often used to achieve higher separation yields. This combination of chemical and physical processes is known as a physicochemical process.
There are alternatives to centrifugation for treating effluents with high sludge loads, such as separation using filter presses or belt filters, which use filtering fabrics to separate solids.

Separation of contaminants by size
The following processes are used for the separation of larger contaminants:
- Simple filtration systems in sand beds.
- Dual sand/anthracite systems.
- Diatomaceous or mixed systems.
These are physical separation technologies that use percolation to retain solid contaminants in the filtering bed. These filtration systems can achieve reliable separation levels of 50 – 100 μm.
To filter particles of smaller sizes, separation technologies using microfiltration, ultrafiltration, and nanofiltration membranes are employed. When filtering at these levels, measurement units such as microns and Daltons are used.

Separation of contaminants by diffusion
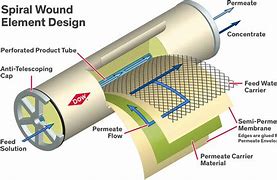
When it is necessary to reduce the saline load of wastewater, more sophisticated treatments can be chosen, such as diffusion through semipermeable membranes, as in the case of reverse osmosis, dialysis, or electrodialysis. These technologies reduce total salinity (TDS) by over 99%. The dimensional measurement unit is the Armstrong, but in practice, the conductivity (μS/cm) of the treated water, referred to as permeate, is measured.
Filtration technologies (microfiltration, ultrafiltration, and nanofiltration) take advantage of the size difference of the particles to be separated in relation to the pore size of the membrane. This is not the case with reverse osmosis.
Reverse osmosis takes advantage of the diffusion or permeability that contaminating substances have to cross a membrane. While the solvent permeates the membrane relatively easily, suspended and dissolved particles cannot do so, or find it very difficult.
This same principle also applies to dialysis and electrodialysis processes, although in the latter, the application of an electric potential acts as a driving force that allows for separation.
Separation of salts by ionic charge
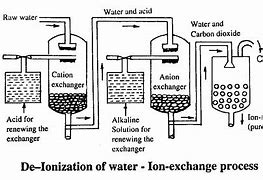
Ion exchange resins are another efficient and popular technology for the separation of salts, as they achieve high-quality demineralized water (conductivity ≤ 0.1 μS/cm).
To obtain ultrapure water, so-called mixed beds (MB) of cationic and anionic resin are used, or mixed systems of membranes and resins known as Electrodeionization (EDI).
In the case of ion exchange, the ionic charge allows for differences to be established between ions. For example, a cationic resin will exchange ions with a positive charge (cations) without interacting with ions with a negative charge (anions).
Separation by vapor pressure
Distillation separates and condenses each compound by boiling the wastewater. The different contaminants are separated at different points of evaporation, condensation, and vapor pressure.
This process is based on the different vapor pressures of the pure substances that make up the mixture to be treated. The greater the difference between the vapor pressures of the different components of the mixture, the more easily the substances will separate through distillation.
Separation by solubility
The different solubility of a solute in one fluid or another allows for effective separation through absorption or extraction.
Separation by surface area
Activated carbon provides high surfaces with high surface tension due to its morphological porosity and micro charge differentials. These characteristics favor the adhesion of small particles.
Separation of contaminants by vacuum evaporation and crystallization
Vacuum evaporators achieve the boiling of wastewater at low temperatures by operating in a vacuum. This allows for significant energy savings, while contaminants are separated and dehydrated until they are crystallized in what are known as vacuum crystallizers. This process yields higher quality condensed water.
Vacuum evaporators stand out in the treatment of complex discharges, as they allow for high concentrations of separated materials, which are sometimes recoverable.
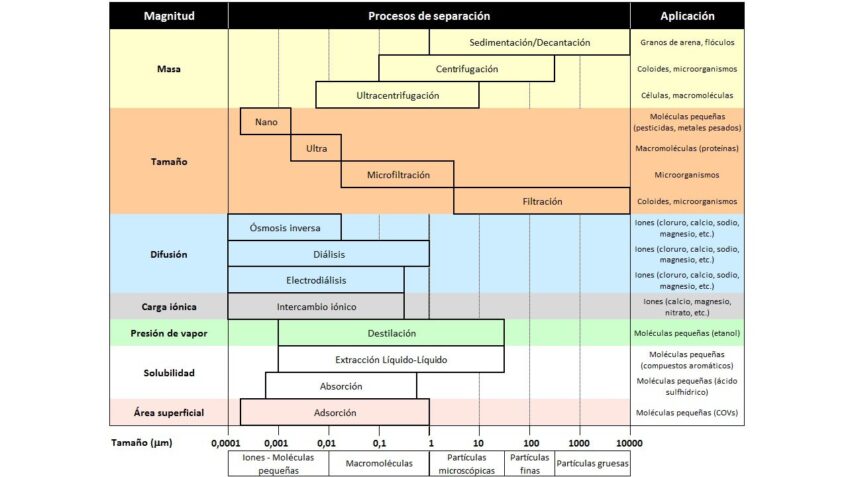
The following table presents the different methods and technologies for separating contaminants present in wastewater:
Concentration of separated contaminants
The residues separated by the technologies analyzed in this article have concentrations that vary between 1 – 4%, except for those separated in screens and sieves, which have higher concentrations.
Most of these contaminants must be sent to authorized managers, so it is important to minimize their volume to reduce management and transportation costs. Additionally, and with few exceptions, landfills do not accept waste with concentrations lower than 30% in MS, so these must undergo concentration processes using various dehydration systems.
The most common processes for reducing their volume are mechanical filtering systems such as filter presses and belt filters that use filtering fabrics to concentrate the waste, or centrifuges that concentrate them by centrifugal force. These residues are usually subjected to coagulation and flocculation processes to improve the performance of mechanical drying.
As indicated in the previous section, there are other systems for separating and concentrating contaminants, such as evaporation and vacuum crystallization. These technologies are the most efficient in minimizing the waste that must be sent to managers, while also allowing for the recovery of high-quality condensates that, in many cases, can be reused as by-products, contributing to reducing the operating costs of wastewater treatment plants.
Evaporation and crystallization technologies are particularly efficient when there are economical energy sources available. For example, when there is surplus energy available such as steam or hot water, or when renewable energy sources are available.

The moisture of the resulting waste can be reduced to near dryness, resulting in lower transportation and disposal or handling costs.
The use of crystallizers is usually limited to the recovery of valuable products or to the maximum concentration of hazardous contaminants.
Summary
Wastewater contains contaminating substances that are found in the natural environment or that are contributed by human activities. These contaminants must be separated to use this water in specific processes or to return it to the environment, for which different treatment techniques are used depending on the type of substances and the destination of the treated water.
The most commonly used processes range from simple screening to systems for obtaining ultrapure water through ion exchange and semipermeable membranes.
The separated contaminants are in the form of solid waste or sludge, and in most cases require conditioning and dehydration to be sent to managers.
In the case of recoverable waste, vacuum evaporation and/or crystallizers are used to minimize their volume and cost while recovering a high-quality condensate.