What is crystallization?
Crystallization is a separation method in which a solid (crystal or precipitate) is formed from a homogeneous phase, either liquid or gaseous. The solid obtained can be very pure, which is why crystallization is used at an industrial level as a purification process. On the other hand, the crystallization of water containing salts, or brine treatment, is a very common application in industrial processes that generate wastewater.

For crystallization to take place, it is essential that the solution is supersaturated. Crystallization processes differ from one another in the method used to achieve supersaturation. In general, there are three main ways:
- Supersaturation produced by cooling the solution with negligible evaporation.
- Supersaturation produced by solvent evaporation with little cooling.
- Evaporation by combining cooling and evaporation in adiabatic evaporators (vacuum crystallizers).
It should be noted that to use crystallizers in which supersaturation is achieved by cooling, solutes must have a solubility curve that decreases significantly with temperature. In cases where solubility hardly depends on temperature, supersaturation is achieved by evaporating the solvent. And when combining cooling and evaporation, a solution is subjected to vacuum conditions so that the solvent evaporates suddenly and the solution cools adiabatically.
This last method is the most commonly used in industry to induce supersaturation. In practice, there is a wide variety of industrial crystallizers, each specifically designed to optimally achieve the supersaturation of the solution depending on its characteristics and properties.
How does the crystallization process work?
The crystallization process is not simple, and the most important stage consists of forming solid crystals within the liquid solution. The solution is concentrated and cooled until the solute concentration is higher than the solubility at that temperature, and the solute forms almost pure crystals.
The growth rate of a crystal is known as the crystallization rate. Growth first occurs with the formation of the nucleus and then continues gradually. When the concentration exceeds supersaturation, nucleation –the formation of nuclei– occurs naturally, spontaneously, and rapidly. The crystallization rate can be expressed by the following empirical equation:
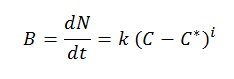
Where:
- B: nucleation rate (nuclei formed per unit of time and solvent volume)
- N: number of nuclei formed per unit of solvent volume
- t: time
- k, i: empirical parameters
- (C-C*): supersaturation
- C: solute concentration in the solution
- C*: solute saturation concentration
From the equation it follows that the nucleation rate is directly dependent on supersaturation. It has been shown that when supersaturation is high, both the nucleation rate and the crystal growth rate are also high, resulting in small, imperfect crystals with impurities. Conversely, when supersaturation is low, the formation rate is small, crystal growth is regular, and the resulting crystals are large and highly pure.

Crystallization mainly involves two steps: nucleation and crystal growth. Both processes occur, if conditions are favorable, in the supersaturated area of the graph. However, nucleation requires more supersaturation than growth. The nuclear formation area where supersaturation occurs is called the unstable zone, while the growth area is known as the metastable zone. For nucleation, the solution must reach the unstable zone. However, once there, nuclei grow too quickly, and the resulting crystals are numerous and very small. To obtain the largest and purest possible crystals, it is necessary to control the number of nuclei forming. If the solution contains no impurities or crystals of its own type, the nucleus can only form by homogeneous nucleation. If some foreign particles are present, nucleation is facilitated, and the process is known as heterogeneous nucleation. Both homogeneous and heterogeneous nucleation occur in the absence of crystals of the same solution and are collectively known as primary nucleation. Secondary nucleation refers to the process of crystal formation that is conditioned and caused by the presence of particles of the same phase in the supersaturated system.
Applications of crystallization
The crystallization process has numerous industrial applications, and obtaining pure crystals is not always the goal. Crystallizers are often used as part of a broader treatment of liquid effluents. In this case, the main objective is the separation of contaminants present in an effluent from the solvent itself, so that the pure solvent is obtained and the contamination is converted into a solid form, making its management more economical. For example, this crystallization application is essential in zero liquid discharge processes, in which the effluent is separated into two streams: one of relatively pure solvent suitable for reuse, and another of contamination in solid or semi-solid state.
Thus, crystallization also stands out as an excellent solution in cases where the main objective is not to obtain a solid product of high purity, as in the following applications:
- Treatment of effluents with a high pollutant load
- Treatment of effluents when conventional techniques are not effective (such as brines)
- Inability to discharge treated effluents
- Treatment of effluents with highly variable composition
Nowadays, crystallizers for the treatment of waste brines are highly cost-effective, Energy consumption has been optimized and reduced, while they remain a very durable and reliable technology in their operation.