Sections
- What is ion exchange?
- Ion exchange applied in wastewater
- Vacuum evaporation and concentration of waste from ion exchange
- Conclusions
What is ion exchange?
There are discharges that, due to their nature and composition, are difficult to treat and adapt to current regulations. This happens when we encounter certain cations or anions that are hardly separable by conventional systems such as physicochemical treatments, biological treatments, and even high-salt-rejection separating membranes.
This occurs, for example, with nitrates, boron salts, fluorine, arsenic, and other heavy metals such as lead, cadmium, or mercury, which are particularly toxic.
Ion exchange is a process in which a material called exchange resin is used, which is capable of selectively retaining dissolved ions on its surface, temporarily holding them to the surface, and releasing them using regenerating solutions.
The exchange reaction is schematized as follows:
(a) RNa+ + Ca++ ——> RCa++ + Na+
In this case, the regeneration of the resin would be carried out by adding an excess of regenerating solution (NaCl).
(b) RCa++ + Na+ ——-> RNa+ + Ca++
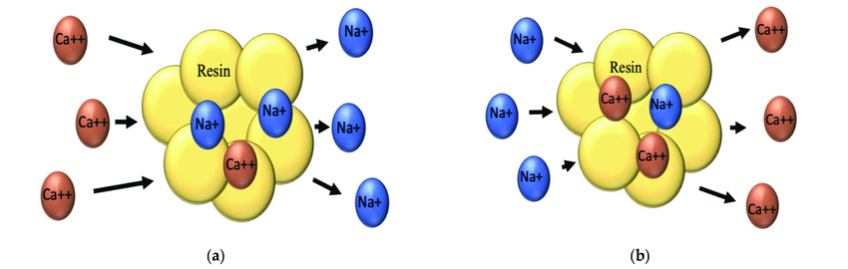
Ion exchange resins are divided into two basic types, according to their polarity:
- Those with a negative charge and, therefore, applicable for exchange with anions, are called “anionics.”
- Those with a positive charge are called “cationics”; they appear as small spheres of plastic material (like Bakelite) with a diameter of about 0.5 – 1 mm.
A common application of ion exchange resins is the removal of salts when they are present in low concentrations.
This is very common in the processes of demineralization and softening of water or other solutions.
Ion exchange is also efficient for the retention of certain chemicals.
The main characteristics of this process are as follows:
- The resins act selectively, so they can prefer one ion over another with relative affinity values of 15 or more.
- The ion exchange reaction is reversible, meaning it can proceed in both directions. (Exchange / regeneration)
- Electroneutrality is maintained in the reaction.
There are natural substances, such as zeolites, that have exchange capacity, but in water treatment, synthetic polymeric resins are used, as they offer significant advantages.
Among the advantages of the ionic process for water treatment, the following stand out:
- They are very versatile equipment as long as they work with relatively low salt concentrations.
- Ion exchange resins are a compact system with high treatment capacities.
- Low economic cost compared to other technologies.
- The resins are very chemically stable, long-lasting, and easy to regenerate.
- There is some ease of automation and adaptation to specific situations.
Ion exchange applied in wastewater
In the case of wastewater, the concentration of the salts to be removed must be taken into account, as the resins are not cost-effective for water with high salinities, since their retention capacity is limited (approximately 50 g CaCO3/ l resin). A high concentration of salts would imply the use of a large amount of resin, as well as significant volumes of regenerating reagent.
We must also consider that, despite the high resistance of the resins to acidic and basic media, they are easily damaged by the presence of oxidants such as Cl2 and by high temperatures.
Ion exchange resins are also sensitive to fouling and the presence of organic matter, so pretreatment is essential for their proper application.
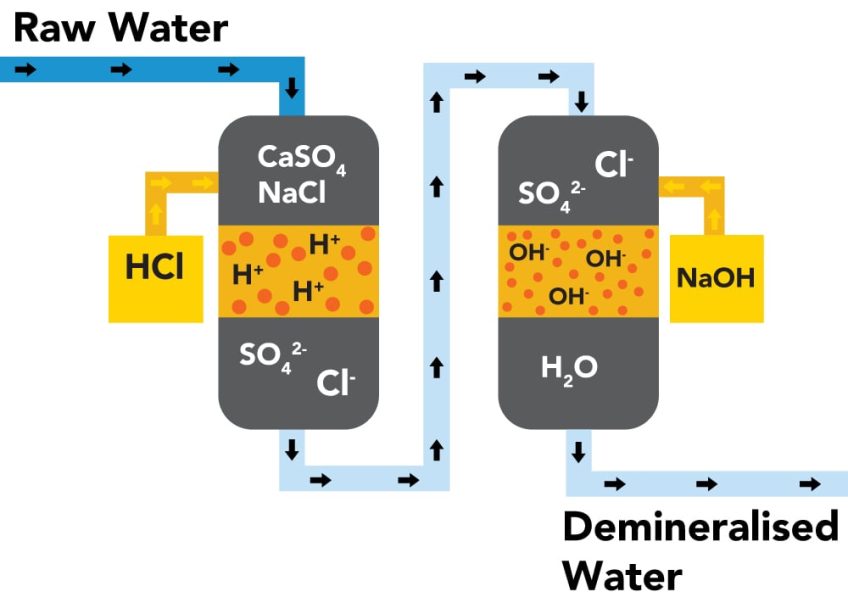
Selective resins
Ion exchange resins have emerged as an effective solution for the selective removal of ions and contaminants. As mentioned, these resins can be of two types:
- Cationic type when their polarity is positive and they are capable of separating cations such as lead, cadmium, and mercury.
- Anionic type if they have a negative charge and are capable of separating anions such as nitrates, sulfates, arsenate, chromate, among others.
Some resins can be modified to increase their affinity for specific compounds, that is, to improve their effectiveness in separating certain contaminants.
Advantages of Using Selective Resins
- High Efficiency: Ion exchange resins can achieve high levels of purification, removing up to 99% of contaminants.
- Selectivity: They allow the specific separation of toxic ions without affecting other beneficial elements in the water.
- Recyclability: These resins can be regenerated and reused, reducing costs and waste.
Applications in Wastewater Treatment
Ion exchange resins are used in various applications:
- Treatment of industrial effluents: Especially in industries such as mining and metal manufacturing, where effluents contain heavy metals and other toxic compounds.
- Desalination: In saline water treatment processes, where it is crucial to remove sodium and chloride ions.
- Nutrient separation: In urban wastewater treatment systems, to reduce nitrogen and phosphorus levels.
Despite their advantages, the use of ion exchange resins faces certain drawbacks:
- Cost: The initial investment in high-quality resins can be high.
- Regeneration: The regeneration process generates effluents that require additional treatment. The new effluent contains the contaminants separated by the resins, along with excess regenerants.
- Lifetime: The lifespan of the resins can be affected by extreme conditions or contaminants in the water.
Cheating resins
This type of resin contains specific functional groups, which give it a selective superiority towards certain special metals. These resins have a wide range of applications, among which it is worth highlighting:
- Decalcification of brine in the Chlor-Alkali industry
- Pulp resin for metals, copper, nickel, zinc, and cobalt.
- Boron separation in drinking water.
- Recovery of precious metals.
- Removal of mercury.
Vacuum evaporation and concentration of waste from ion exchange
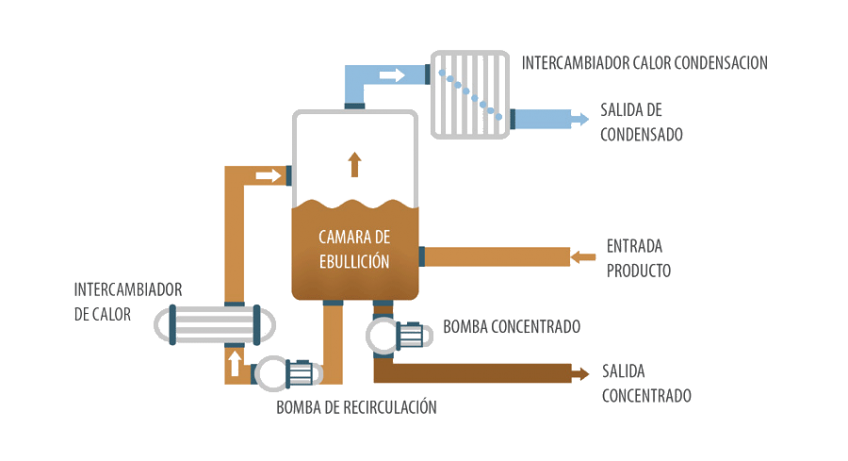
A vacuum evaporator is equipment that allows the concentration of liquid waste by evaporating its aqueous component under vacuum conditions.
This process reduces the pressure in the evaporation chamber, which decreases the boiling point of water, allowing it to vaporize at lower temperatures. This is especially useful for concentrating effluents from the regeneration of ion exchange resins.
The concentration process using vacuum evaporators is based on the use of a vacuum pump to reduce the pressure inside, allowing water to evaporate at lower temperatures.
As heat is applied, the water vaporizes and separates from the dissolved or concentrated solids in the effluent. The generated vapor is directed to a condenser, where it is cooled and converted back into liquid, which can be reused or treated.
The remaining solids and other compounds are concentrated at the bottom of the evaporator, ready to be properly managed.
Advantages of Vacuum Evaporators
- Energy Efficiency: Operating at low temperatures, vacuum evaporators require less energy compared to conventional evaporation systems.
- Protection of Sensitive Compounds: This technology minimizes the decomposition of substances that could be degraded at high temperatures.
- Water Recovery: The condensed water can be recovered and reused in the process, contributing to the sustainability of the system.
- Reduction of Waste Volume: The concentration of effluents significantly reduces the volume of waste to be managed, facilitating subsequent treatment.
Despite their numerous advantages, it should be noted that the cost of this equipment and its energy consumption are relatively high, making these devices more suitable for small volumes with high concentrations of contaminants, such as in the case of effluents from the regeneration of ion exchange resins.

Conclusions
The ion exchange process is complementary to conventional treatments (physicochemical, biological, etc.) and allows for the separation of certain cations and anions from water based on the affinity for ions in solutions, which can be regenerated with suitable substances (regenerants).
Selective ion exchange resins (chelating) are used in wastewater treatments that contain complex and toxic contaminants.
Vacuum evaporators are an efficient and sustainable solution for concentrating effluents from resin regeneration. Their ability to operate under vacuum at low temperatures and their high efficiency make them an excellent option in numerous industries.