Transformation of waste into fertilizers
Livestock farms, dairy farms and poultry farms produce a huge amount of wastewater which contains high volumes of organic waste.
Other wastewater treatment processes also generate sludge, which can also be treated to obtain valuable byproducts, including fertilizers, which can be reused in agricultural activities as fertilizer or for composting.
The valorization of waste through its transformation into a valuable product, such as fertilizers or clean water, is a clear example of circular economy.
We design and build sludge and digestate management plants that transform waste into reusable resources and by products, such as:
- Clean and reusable water.
- Fertilizers.
- Biogas that can be used to generate thermal energy.
The most widely collected byproducts for transformation into fertilizers are:
- Sludge from aerobic wastewater treatment processes.
- Digestate produced in biomethanization plants.
It is important to note that sludges from wastewater treatment may contain pathogenic germs and parasites dangerous to humans, such as salmonella, Escherichia coli, ascaris, etc. Therefore, their use is regulated by directives.
Our system offers numerous environmental and economic benefits:
- We provide a solution to an environmental problem by transforming a waste product that is otherwise difficult and expensive to treat into a source of self-sufficiency and revenue for farms.
- The self-supply of water, energy and fertilizers coming from wastewater and sludge reduces the operational and waste management costs.
- Self-production of ecologically friendly and high-quality fertilizers that can be sold if you have a surplus. Revenue can be also generated from the sale of surplus energy.
This circular process is based on the recycling of products, optimizing the use of mineral resources, recovering and incorporating the by-products produced, minimizing emissions and reducing dependence on non-renewable energy sources.
Treatment and Valorization of Digestate
The biodigestion process is mainly carried out to produce biogas, which can be used as a fuel, from various organic wastes, including animal excreta.
In addition to biogas production, biodigestion also reduces the pollutant potential of the excreta used to generate biogas, reducing chemical oxygen demand (COD) and biological oxygen demand (BOD) by up to 90%.
As a result of this process, a residue called digestate is generated, which has a high concentration of nutrients and organic matter, making it ideal for use as fertilizer.
Digestate is a semi-liquid byproduct and can be applied directly or after separation into two fractions, solid and liquid, which increases its effectiveness.
The advantages of transforming digestate into a fertilizer include:
- Compared to organic waste before digestion, digestates are more suitable for agricultural use, generate fewer odors, and have a higher hygienic quality.
- Digestate undergoes a higher degree of mineralization as organic nitrogen and phosphorus are converted to minerals during fermentation. This makes it comparable to mineral fertilizers. The increase in prices of the latter presents an opportunity for digestates.
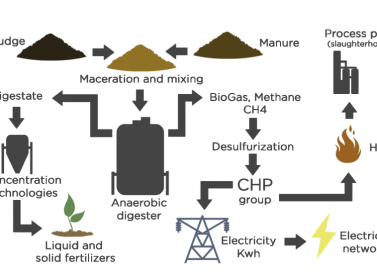
Process diagram for manure and digestate treatment
The following diagram shows the main processes and technologies involved in a plant designed to turn the waste generated in farms into valuable resources and by products.
1. Digestate treatment. The waste products (liquid sludge (*) + animal manure) is sent to the raw materials tanks for storage and mixing.
2. Anaerobic digestion of manure. The waste mixture is sent to the anaerobic biological digesters for treatment, resulting in the extraction of biogas and a digestate, from which a liquid fraction is separated (88%) and a solid one (2%).
3. Biogas production and treatment in farms. The biogas is sent to the co-generation plant to be converted into electricity and heat. Alternatively, biomethane can be produced by purifying the biogas.
4. Concentration and fertilizer production. The liquid fraction of the digestate, which contains valuable organic nutrients and minerals, undergoes several concentration phases by means of mechanical and thermal systems, thanks to which a concentrated liquid fertilizer is obtained which can be sold as well as clean water that can be discharged or re-used.
Technologies for Digestate Treatment
Once slurries have been mixed with biomass and transformed into biogas in a biodigester, this biogas is converted into electrical energy in a combustor. After this valorization process, an effluent is obtained that cannot be discharged due to its high concentration of ammonia salts, known as digestate.
Mechanical separation processes followed by a membrane system can separate the solid part, which can be used as fertilizer, from the liquid part, which must be managed as waste.
The problem with this process, without adding any additional stages, is that it does not produce the highest-quality fertilizer since it is not fully effective in separating liquids from solids and concentrating organic matter.
This lower-quality fertilizer has two disadvantages:
- Wastage of a significant portion of solids and organic matter in digestate, which cannot be separated, resulting in a much smaller quantity of fertilizer and reducing potential revenue from its sale.
- Increased waste volume to be treated, as all the material not used to produce fertilizer becomes waste, incurring economic costs—turning potential income into expenses.
For these reasons, it is more profitable and efficient to reduce the volume of digestate in the same plant and recover the significant amount of fertilizer that is lost.
To achieve this, digestate is treated in a vacuum evaporator, which allows the recovery of 97% of clean water while obtaining a concentrate that can be converted back into stabilized fertilizer in a composting tank.
A vacuum evaporator can separate between 90% and 99% of the water from solids and organic matter, allowing for a highly concentrated fertilizer.
The increased fertilizer production and the savings resulting from sending a smaller quantity of waste to disposal more than compensate for the initial investment in acquiring the vacuum evaporator, making it the most cost-effective and efficient solution in the medium term.
Its advantages include:
- Increased fertilizer recovery and significant cost savings by not sending liquid digestate to waste disposal.
- Clean water (condensate) obtained during the evaporation process can be used as mixing water at the beginning of the biogas process or safely discharged if preferred.
- The combined fertilizer product can still be dried further and converted into dry fertilizer.
An evaporation plant is more efficient at removing water from digestate than drying it in a drying plant.
Increased fertilizer sales improve the profitability of the biogas plant. - Digestate is a substance that fouls equipment, which lengthens downtime. Scraped surface evaporators ensure uninterrupted operation.
- Evaporators can concentrate digestate to high levels of density, producing highly viscous concentrates.
Case studies
| CASE #1: PRODUCTION FIGURES | CASE #1:ECONOMIC BALANCE SHEET | ||
| DESCRIPTION | VALUE | DESCRIPTION | VALUE |
| Volume of waste water treated (m3/yr) | 2,200 | Value of electricity generated (USD/yr) | 437,400 |
| Volume of primary sludge generated (m3/yr) | 29,200 | Value of calorific energy (USD/yr) | 257,760 |
| Volume of chicken manure (Tn/yr) | 15,000 | Value of fertilizers (USD/yr)(Tn/yr) | 532,280 |
| Biogas production from sludge (m3/Tn) | 50 | Savings in sludge management(USD/yr)(m3/Tn) | 187,500 |
| Biogas production from manure (m3/Tn) | 70 | Total income (USD/yr) | 1,414,940 |
| Volume of biogas from sludge (m3/yr) | 1,460,000 | Operating Expenses (USD/yr) | 329,481 |
| Volume of biogas from manure (m3/yr) | 1,125,000 | Economic return (USD/yr) | 1,085,459 |
| Total volume biogas (m3/yr) | 2,585,000 | Project investment (USD) | 4,200,000 |
| Volume of methane (m3/yr) | 1,581,000 | Period to break-even point (years) | 3.9 |
| Electricity generated (KWh/yr) | 4,374,000 | ||
| Calorific energy production (KWh/yr) | 6,444,000 | ||
| Fertilizer production (Tn/yr) | 3,550 (*) | ||
| CASE #2: PRODUCTION FIGURES | CASE #2: ECONOMIC BALANCE SHEET | ||
| DESCRIPTION | VALUE | DESCRIPTION | VALUE |
| Volume of dilution water (m3/yr) | 43,800 | Value of electricity generated (USD/yr) | 1,288,369 |
| Volume of chicken manure (Tn/yr) | 74,825 | Value of calorific energy (USD/yr) | 498,490 |
| Biogas production from manure (m3/Tn) | 73 | Value of fertilizers (USD/yr) | 2,353,500 |
| Volume of biogas from manure (m3/yr) | 5,479,613 | Total income (USD/yr) | 4,140,359 |
| Volume of methane (m3/yr) | 3,013,788 | Operating Expenses (USD/yr) | 740,000 |
| Electricity generated (KWh/yr) | 12,883,695 | Economic return (USD/yr) | 3,400,359 |
| Calorific energy production (KWh/yr) | 12,462,266 | Project investment (USD) | 7,800,000 |
| Fertilizer production (Tn/yr) | 15,690 (*) | Period to break-even point (years) | 2.3 |
Other Options for Transforming Sludge into Fertilizers
For the recovery of phosphorus in the form of solid fertilizer, it can be crystallized as struvite, a slow-release mineral fertilizer composed of magnesium, phosphorus, and nitrogen, with low metal content. Struvite gradually releases nutrients to the soil, promoting plant absorption and reducing surface losses that can end up in water bodies.
Additionally, the production of liquid fertilizers rich in ammonium can be feasible through an adsorption-desorption process with zeolites and membrane contactors. In some cases, achieving a specific ratio of primary nutrients may require the removal of excess nitrogen, which can be accomplished through a process under microaerophilic conditions with low energy consumption.
Another waste-to-fertilizer transformation method involves mixing the waste with other organic residues and mineral fertilizers to adjust nutrient levels (N/P/K) to commercial values. Subsequently, the resulting mixture is introduced into a vacuum evaporator to remove water and transform the material into a stable and easily usable solid.
Circular Economy in Fertilizer Production
The application of fertilizers is an essential agricultural practice with the primary goal of maintaining soil fertility. It should not only aim to replace the nutrients extracted by crops but also address the nutrients lost through leaching, retrogradation, and erosion.
Fertilizers allow for the replenishment of these extracted essential nutrients, making them available to crops.
Complex NPK fertilizers are products containing two or three primary nutrients (N, P, K) and may also include secondary nutrients (Ca, Mg, S) and micronutrients (Zn, Cu, B, etc.). They are applied to balance and enhance soil nutrient content, considering the needs of the intended crop and expected yield. Fertilizers can be in solid (granular) or liquid form, with the latter becoming increasingly common.
Currently, there is a growing demand for fertilizers along with the generation of a significant amount of waste, much of which contains valuable nutrients (N/P/K). In this context, it is necessary to promote circular economy systems that enable fertilizer production from waste, valorizing and incorporating byproducts into the cycle, minimizing emissions, and reducing dependence on non-renewable energy sources.
Treatment of Sludge with Quicklime
Studies have shown that the addition of quicklime to these sludges eliminates pathogens. Adding lime to sludge reduces odors and the level of pathogens by creating a high pH that is hostile to biological activity. The gases released during the anaerobic decomposition of organic matter contain nitrogen and sulfur and are the main source of foul odors from sludge. When lime is added, the microorganisms involved in decomposition are strongly inhibited or destroyed in this highly alkaline environment. Pathogens undergo a similar process.
During the process of sludge treatment with quicklime, it is necessary to maintain the pH above 12 for a minimum of 2 hours to ensure the destruction of pathogens and provide sufficient residual alkalinity to prevent the pH from dropping below 11. This allows enough time for storage or disposal of stabilized sludge.
The amount of lime required to stabilize sludge depends on its composition, solids concentration, and retention time. Roughly, the range varies from 6% to 51%. Primary sludges require the least amount of lime, while activated sludges require the most.
There are other sludge treatment methods, such as aerobic and anaerobic digestion, but lime treatment provides greater advantages when it comes to reuse since it yields a larger volume of usable product and provides the necessary neutralization for acidic soils at no additional cost.
The high lime dosing also affects the physical and chemical characteristics of the sludge. These reactions result in a reduction of nitrogen, acting as a limiting factor for the amount of sludge that can be applied to the soil. This allows for a larger quantity of sludge per unit area, while improving moisture loss capacity and the characteristics of secondary liquid fluids.
Another advantage of this system is that it can be a good alternative when additional sludge treatment methods are needed. Lime stabilization can be initiated and completed quickly. Therefore, it can supplement existing facilities when sludge volumes exceed design levels, replace incineration during fuel shortages, or during maintenance activities.
This sludge treatment method is more cost-effective than other methods, and it provides an effective and safe means for the final disposal of sludge, avoiding risks to human health and environmental damage. Once the sludge has been treated and stabilized, it can be safely discharged. It is ideal for agriculture, as its high lime content makes it an excellent quality fertilizer for acidic soils, containing organic materials and nutrients.