Sections
- Condorchem Envitech offer
- Introduction
- Uses of lithium
- Lithium extraction
- Precipitation and refining of lithium chloride
- Lithium recovery solutions
Condorchem Envitech offer
Condorchem Envitech offers a set of high-efficiency solutions for the extraction and concentration of lithium. Our main objective is to offer a competitive and profitable solution that allows mining operations to meet the increase in demand for lithium that they have experienced in recent years:
Our solutions for the extraction and concentration of lithium are divided into the following processes:
- Design of a customized lithium salt extraction process
- Extraction and physicochemical purification of lithium effluents
- Concentration and crystallization of lithium salts
- Laboratory tests: Matter balance and efficiencies
- Demonstration industrial pilot plants
On the other hand, we also offer comprehensive solutions for the recovery of lithium, as well as other metals, in recycling processes for all types of batteries.
Introduction
Lithium is found in nature in around 145 minerals, but in only some is it available in commercial quantities. It also occurs in brine, hot springs and sea water in amounts between 20-65 ppm.
The element can be found in many different forms: for example, in abnormal concentrations of pegmatites; in sedimentary environments associated with clay; in areas of hydrothermal alteration associated with minerals at low and high temperatures; in non-marine evaporites; in brine from desert environments; in saline or brine associated with oil fields; in deposits of boron, beryllium, fluorine, manganese and possibly phosphate; in lake environments associated with magnesium silicates; in waters, plants and soils in desert environments; and in iron-rich sedimentary rocks.
In addition to the above, the main deposits in operation are located in pegmatites or in paleobrine and saline lake deposits, with the vast majority of prospecting factors verifying the presence of only anomalous concentrations of lithium, not currently economic viable.
In Chile, lithium is found in the saline deposits of the Alta Cordillera and, to a lesser extent, in nitrate fields and associated salt deposits.
Lithium has a number of applications: In the manufacture of batteries for computers, mobile phones and electric cars (the demand for these is expected to increase dramatically when production becomes mass-produced); specific pharmaceuticals for nervous disorders (antidepressants); purification of ambient air; aeronautic alloys (Mg-Li); and in lithium-based lubricants for the nuclear industry, as a coolant and pH regulator, and obtaining tritium for future generation nuclear fusion reactors.
Lithium carbonate (Li2CO3)) is the most widely used lithium compound; 5.32 grams of which contains 1 g of lithium.
Chile is the biggest producer of lithium in the world, with known reserves of 4.3 x 106 tons in its largest deposit, the Salar de Atacama, which contains 40% of the economically viable reserves in the world.
After the entry into the market of Minsal (Sociedad Minera Salar de Atacama Ltda) in 1998, Chile became the world’s leading producer and exporter of lithium: producing 30,000 tons of concentrate, equivalent to 50% of world market demand, with the Sociedad Chilena del Litio (SCL) leading the exports of this mineral.
In Japan alone, papers are published containing studies of around 10,000 new materials every year, with different physical, chemical, electrical, magnetic, ionic and electrochemical properties. The development of new products, such as cyanide, hydroxide and lithium metal, is ongoing.
Uses of lithium
| Usos del litio | |
| Batteries | 35% |
| Ceramics, glass, cement | 32% |
| Lubricating greases | 9% |
| Air conditioning | 5% |
| Metallurgical uses | 5% |
| Polymer synthesis | 4% |
| Primary production of aluminum | 1% |
| Others | 9% |
The first commercial uses of lithium were in metallurgy using small amounts of aluminum-zinc-lithium alloys and lead alloys, in which lithium was added as a hardener.
Between 1953 and 1959, the United States Atomic Energy Commission used large amounts of lithium hydroxide to separate the lithium-6 isotope, used in the development and production of the hydrogen bomb. Since 1961, lithium has been used in compounds such as lithium bromide, in the form of concentrated brine, for absorption air conditioning equipment; lithium carbonate for the ceramic industry; metallic lithium, as an intermediary in the synthesis of pharmaceutical products; and butyl lithium, as a catalyst in the polymerization of synthetic rubber manufacturing.
New markets were developed for various purposes; however, the ceramic industry is still the most important market today, where lithium carbonate is used as a fluxing agent in the preparation of glazed enamels and glass.
From 1974, the use of metallic lithium as an anode in primary batteries began to show rapid growth, as lithium is electrochemically reactive, in addition to possessing other unique properties.
In 1980, the aluminum industry displaced ceramics and glass as the main user of lithium products. The development of Li-Al alloys has led to advances and the development of new uses in research, for the aluminum and aeronautics industries and the military.
As a result, a lighter alloy was achieved by adding 1.5% to 3% of Li to the conventional aluminum alloys used in commercial and military aircraft components; thus producing a material which is 10% lighter, saving fuel and reaching up to 20% of an aircraft’s cargo capacity.
Currently, the consumption of lithium metal for these alloys is around 45 tons annually, or about 500,000 pounds of lithium carbonate. More recently, pyroceramics have been developed that find great applicability in the aerospace industry. As this type of material contains lithium, the expansion and compression properties are almost zero when under extreme temperature conditions.
The United States is still the number one producer of higher value-added lithium compounds and the largest consumer of all types of lithium materials, consuming 2,800 metric tons of lithium in 2000.
Lithium compounds also meet the needs of the primary aluminum industry, are used in battery components, air conditioners, lubricants, dehumidification systems, production of sophisticated textiles, disinfectants for swimming pools and baths and as bleaches in dry laundries.
The crystalline structure of lithium stabilizes only because of the electrostatic attraction between the fixed ions in the lattice sites and the free electrons.
However, since there are few free electrons, the forces of attraction are not very strong and the lithium lattice is weak and easily deformable, causing very low hardness.
It has a low melting point of 180.5°C; however, the amount of heat required at this temperature to destroy the lattice and melt the metal is extremely high.
Therefore, lithium is useful as a heat sink, particularly in systems where a low overall design weight is required, and is of great importance in the nuclear industry as a closed loop reactor heat transfer material.
The ease with which lithium gives up its outer electron means it is a highly powerful reducing agent and reacts quickly with less powerful oxidizing agents.
For example, it reacts with nitrogen at room temperature to form the nitride Li3N; with oxygen in the air it reacts quickly to form the oxide, Li2O; while with fluorine it has the most violent reaction of all elements. Due to these properties, lithium finds applications in very high electrochemical energy production systems, such as lithium-chlorine, lithium-sulfur and various other types of batteries; this is currently an expanding industry.
Lithium is made up of the combination of isotopes, lithium-6 (7.4%) and lithium-7 (92.6%), giving an isotopic atomic weight of 6.941. The 6 isotope is of great importance, as it is the raw material for obtaining tritium which, together with deuterium, are considered as probable nuclear fusion reactor fuels (Tagger 1983); with it being estimated that such reactors will be the solution to global energy problems. The formation of tritium and energy of the reactions are as follows:
3Li6 + 0n1 — 2He4 + 1H3 + 4,78 Mev
The neutrons in turn come from the reaction:
1H2 + 1H3 —- 2He4 + 0n1 R + 17,6 Mev
Where NL and NR are slow and fast neutrons. Only slow neutrons are effective in converting lithium-6 to tritium.
In the nuclear field, PWR type reactors are evaluating the possibility of using another element with a neutralizing and regulating effect in the coolant, other than lithium hydroxide.
Due to the price, which is currently under stress from the demand for lithium in the energy sector, the nuclear industry studied substitution by KOH.
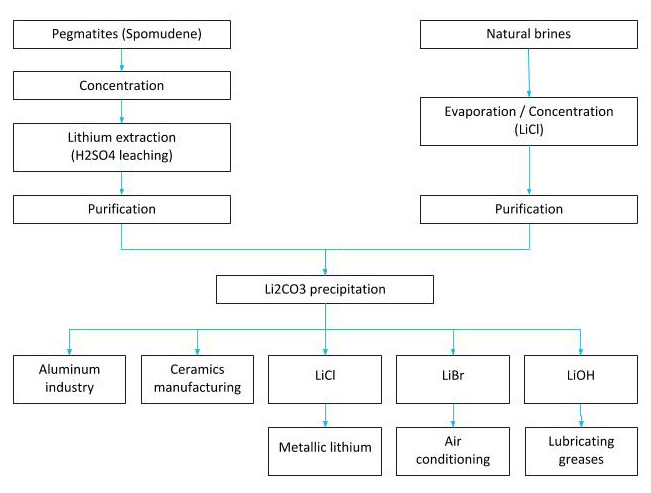
Main lithium sources and extraction processes
Lithium extraction
Lithium is extracted from brine where it exists in natural salts, such as in the Salar de Atacama in Chile and the Salar del Hombre Muerto and others in Argentina; from mineral deposits, such as the Greenbush spodumene in Australia; and one of the largest lithium reserves in Bolivia, 21 million metric tons in the Bolivian salt flat of Uyuni.
Much of the world’s lithium production comes from brine, as the production cost is much less than from mineral deposits ($1,500-2,300/ton and $4,200-4,500/ton, respectively, according to John McNulty).
Lithium is obtained from two natural sources: firstly, the mineral, spodumene, which is aluminum-lithium silicate (LiAISi2O6), and is associated with quartz, mica and feldspar. The other production source is natural brine from salt flats and geysers, mainly in the form of the salt, lithium potassium sulfate (KLiSO4).
As mentioned above, lithium can be obtained from aluminum lithium silicate deposits (LiAlSi2O6 and Li2O·Al2O3·4SiO2), whose content is 3.73% Li and 8.03% oxide, Li2O.
The other related elements are 51.59% O; 30.18% Si and 64.58% as SiO2; 14.5% Al and 27.4% Al2O3. Spodumene (originally from Greece) is also known in America as Kunzite, which is a crystal of hardness 6.5-7 and a density of 3.1 g/cm3. It can be of various colors, such as light gray, yellow, green and purple.
Bolivia’s lithium reserves or resources are in brine, which has an approximate density of 1.2 grams per liter (g/L), so a lithium concentration of 0.1% by weight will be equivalent to 1,000 parts per million (ppm) and a 1.2 g/lL concentration of the lithium salt.
The brine is extracted by pumping and concentrating the lithium by one of two processes.
Firstly, by adsorption using a selective lithium adsorbent (polyethylene glycol); and secondly by evaporation in shallow pools built for this purpose. In addition to raising the concentration of salts, evaporation can also cause precipitation when the solution is saturated.
The advantages of adsorption are that it is not influenced by the composition of the salt water (brine with a low concentration of lithium can be treated experimentally as with seawater) or the meteorological conditions of the location; also, there is little waste produced.
The disadvantages are that reagents are needed, adsorption equipment is expensive and complicated and the adsorbent cost is high.
The advantages of natural evaporation are that no energy and few chemical reagents are used; while the disadvantages are the need to use another separation method at the same time, the accumulation of waste and the dependence on the location weather conditions (evaporation speed and rainfall). The latter method was chosen for the Salar de Uyuni (and a pilot plant is installed), so only a brief description of it will be given.
The world’s largest area for lithium production is from the Salar de Atacama brine in Chile, which uses the evaporation method and for which there are data and operating factors, which can be compared with those of the Salar de Uyuni.
The Atacama brine is richer than Uyuni’s in lithium (also in potassium and boron), so the Mg:Li ratio, harmful for lithium concentration is 6:1 and 19:1, respectively.
Evaporation and rainfall are 3,200 mm/year and 10-15 mm/year in Atacama; while in Uyuni they are 1,500 mm/year and 200-500 mm/year. Thus evaporation is less and the rainfall much higher in Uyuni, which slows evaporation.
In Atacama, the evaporation process concentrates lithium from 0.15% to 6% (40 times) in 12-18 months; so evaporation is expected to take much longer in Uyuni, especially if it rains as hard as it did recently, when it flooded the pilot plant evaporation ponds.
| Main lithium minerals | ||
| Mineral | % Li máx. | % Li commercial |
| Amblygonite | 4.73 | 3.7-4.2 |
| Eucryptite | 5.50 | 2.6-3.0 |
| Lepidolite | Variable | 1.4-1.9 |
| Petalite | 2.26 | 1.4-2.2 |
| Spodumene | 3.73 | 2.6-3.0 |
| Average lithium content in processed brine | ||||||||
| Location | % Li | % Na | % K | % Mg | % SO4 | % Cl | % B | Li/Mg |
| Bolivia: salar de Uyuni | 0.025 | 8.80 | 0.72 | 0.65 | 0.046 | 15.7 | 0.02 | 1/19 |
| Chile: salar de Atacama | 0.14 | 7.6 | 1.87 | 0.93 | 0.03 | 16 | 0.1 | 1/1.64 |
| Israel-Jordan: Dead Sea | 0.0015 | 3.21 | 0.60 | 3.33 | 1.18 | 17.32 | 0.003 | 1/2200 |
| USA: Great Salt Lake, Utah | 0.004 | 8.0 | 0.65 | 1.00 | 0.016 | 14.0 | 0.006 | 1/250 |
| Silver peak, NV | 0.023 | 6.2 | 0.53 | 0.033 | 0.20 | 10.06 | 0.008 | 1/1.5 |
As an example, until 1997, only lithium carbonate was produced from the brine at Salar de Atacama; then, from 1998, it also incorporated lithium chloride in its production process.
Carbonate is obtained from this brine in 2 main stages: Concentration of the solutions in solar evaporation ponds: The initial Li content of the brine at Salar de Atacama is around 0.17%, before reaching concentrations of 4.3-5.8% Li.
Concentrated brine treatment in a chemical plant: To produce Li2CO3 (of 99.5% purity), the concentrated brine is purified and crystallized, before undergoing a carbonation process and subsequent precipitation when the crystals are finally dried.
The lithium recovery process applied by the Sociedad Chilena de Litio (or SCL, belonging to the Foote Míneral Co, a subsidiary of Cyprus Amax Minerals Co) was developed by this company at its plant in Silver Peak, Nevada (USA), and adapted to the properties of this brine.
The production of Chemetall Foote covers the necessary demand for the production of lithium compounds with a higher added value from its chemical plants in the United States, and also meets the needs of the Chemetall parent company in Germany and Taiwan.
Precipitation and refining of lithium chloride
The laboratory study “Chemical treatment of brines from the Salar de Uyuni-Potosí” carried out in 1987 in France by the UMSA-ORSTOM (French Institute for Scientific Research for Development), simulating the conditions of evaporation pools in 5 containers, established that sodium chloride (NaCl) first precipitates and then potassium chloride (KCl) almost immediately.
Since magnesium chloride (MgCl2) cannot be separated with evaporation, which complicates the process, it is precipitated as magnesium hydroxide (Mg(OH)2) by adding lime.
The lithium chloride adequately concentrated in the 5 vessels was washed with sodium hydroxide to remove any remaining traces of magnesium and calcium, before finally precipitating as Cl-using sodium carbonate. The average lithium recovery was 80.8% and the average purity of Cl- was 94.4%.
Three laboratory tests recently carried out with 25 L of brine from Salar de Uyuni with 0.107% lithium, at the National Institute of Advanced Industrial Science and Technology of Japan to obtain lithium by the adsorption method, gave Cl- with a purity greater than 99.8% and an average recovery of 73%.
This method is used in Salar del Hombre Muerto, Argentina, which contains 0.06% lithium.
The Cl- obtained by any method must be purified, dried and crystallized. Despite the high lithium content at Salar de Atacama and the experience gained in processing it, its recovery is 42%.
The Cl- to be used in the manufacture of batteries for electric vehicles must have a purity of at least 99.95%, so the Cl- obtained by precipitation must be refined through various reactions and recrystallization stages, in some cases by an ion exchange resin.
Because the refining process has a cost and its recovery is lower after each stage (in the refining stage it is estimated at approximately 70%), as the purity of Cl- increases, its price increases by a much greater proportion.
Although the method described for obtaining Cl- seems simple, like any industrial process, it requires adequate technology and logistics, experienced technicians and trained personnel.
Evaluation of the pilot plant operation will either confirm the lithium concentration process using evaporation pools or lead to a change.
| Solubilities of Li2CO3 and LiCl in g/100g H2O | ||||||||||||
| Temp. ºC | 0 | 5 | 10 | 20 | 25 | 30 | 40 | 50 | 60 | 70 | 80 | 100 |
| Li2CO3 | 1.52 | – | 1.41 | 1.31 | – | 1.24 | 1.16 | 1.07 | 1.00 | – | 0.84 | 0.70 |
| LiCl2 · 2H2O | 40.9 | 42 | 42.7 | – | – | – | – | – | – | – | – | – |
| LiCl2 * H2O | – | – | – | – | 45.85 | 46.3 | 47.3 | 48.3 | 49.6 | 51.1 | 52.8 | 56.511 |
| Source: Linke & Siedell, 1965 1Hutting and Steudemann, 1927 **Taken by Kraus and Burgess, 1929; density of saturated salt was 1.017 at 0°C and 1.014 a 15°C. | ||||||||||||
Example 1. Process for Li-rich brine in Atacama, Chile
Lithium carbonate is the most demanded base compound among Li salts, representing 60% of Li products.
Its importance lies mainly in the fact that it is easy to purify and can be converted to other inorganic and organic lithium salts, such as, Li2CO3, LiBr, LiOH.H2O and other compounds.
World production is estimated at 45,000 tons per year, with Chile being the main producer, while the main consumer of lithium carbonate is the United States.
The brine from Salar de Atacama has been extracted by the Sociedad Chilena del Litio since 1984, and has a production plant of capacity 11,800 tons per year of Li2CO3.
The salt flat brine, see Figure 7, is extracted by pumps that reach a depth of 30 m then piped to a system of solar evaporation ponds, where the Li is concentrated from 0.17% to 4.3%.
The ponds inside the salt flat were constructed by breaking the salt crust and leaving a flat surface upon which a layer of clay lies. Both the dykes and the bottom of the evaporation ponds were lined with resistant plastic sheeting of 0.5 mm thickness.
The polyurethane protection is achieved with a layer of NaCl salt approximately 30 cm thick. During the evaporation process, salts precipitate in the ponds sequentially, and are harvested and discarded as impurities: halite (NaCl), silvinite (NaCl + KCI), carnallite (KMgCl3 6H2O) and bischofite (MgCl2.6H2O).
Lithium carnalite (LiCl.MgCl2.7H2O) precipitates in the higher concentrations ponds. The lithium it contains is recovered by repulping and washing with a saturated solution of magnesium chloride, but not saturated with lithium chloride.
Bischofite (MgCl2. 6H2O) is present and undissolved; it is separated by centrifugation and then removed from the system. This concentrated brine finally contains 5.8% Li, 20% Mg and 0.7% boric acid (H3BO3), and is ready to be transported to the chemical plant in Antofagasta, 170 km from Salar de Atacama.
The chemical treatment at the La Negra Plant consists of removing the remaining magnesium in two purification stages, as carbonate and magnesium hydroxide, respectively.
This is done by diluting the concentrated brine to a content of 0.6% Li, with the mother liquor from the final stage of lithium carbonate precipitation.
This product is obtained by hot reaction (around 85°C), between the purified, magnesium-free brine (1 ppm) and a Na2CO3 solution, precipitating the Li2CO3. The final product is dried and sold in crystals (70%) or compacted and sold in granules (30%).
The purity of the product is close to 99.5% Li2CO3. However, its boron content (400-600 ppm) prevents its use as a raw material for the manufacture of lithium metal, via lithium chloride.
2LiCl + Na2CO3 == Li2C03 + 2NaCl
To solve this problem and obtain lithium carbonate with the highest specifications required by the market, FOOTE designed a process to remove boron from the brine by solvent extraction, in a stage prior to the separation of the remaining magnesium.
The liquid-liquid extraction unit first separates the remaining boron from the salt flat’s concentrated brine and then continues with the process as described above. Finally, the lithium carbonate product is obtained with a very low boron contents (less than 5 ppm).
In the first stage, most magnesium is leached and the process leads to the formation of a lithium chloride solution with a low sulfate content, before finally being treated with sodium carbonate to obtain lithium carbonate.
In 1998, Minsal estimated its production was 9,000 tons of Li2CO3 with a capacity of over 20,000 tons/year. This company has been considering expansion to build a butyl lithium plant in Texas to facilitate the production of battery materials.
The process developed by Minsal is very different in its early stages from that used by SCL, as it considered using salts harvested from ponds containing lithium sulfate as raw material.
In 1997, SQM, the leader in the commercialization of saltpeter, began commercialization and production of lithium carbonate from Salar de Atacama brine.
A fraction of the brine resulting from the solar evaporation process for the production of potassium chloride continues its concentration process and constitutes the source of lithium from which SQM produces lithium carbonate in a plant at Salar del Carmen.
The lithium-concentrated brine is transported by truck from Salar de Atacama to the plant, where it is purified by first extracting its remaining boron content and then magnesium through extraction and filtration processes.
Finally, the purified lithium brine reacts with sodium carbonate to produce lithium carbonate which is filtered, washed, dried and packaged in different product formats ranging from fine products, such as those used in the rechargeable lithium ion battery industry, to granular products used in the aluminum production process.
Both companies, SQM and FMC (SCL), transport the concentrated brine from the salt flat to their plants located in Antofagasta and practically cover the entire American market (88% of the lithium is imported by the United States from Chile, 7.5% from Argentina, with the rest being small amounts from China and Japan).
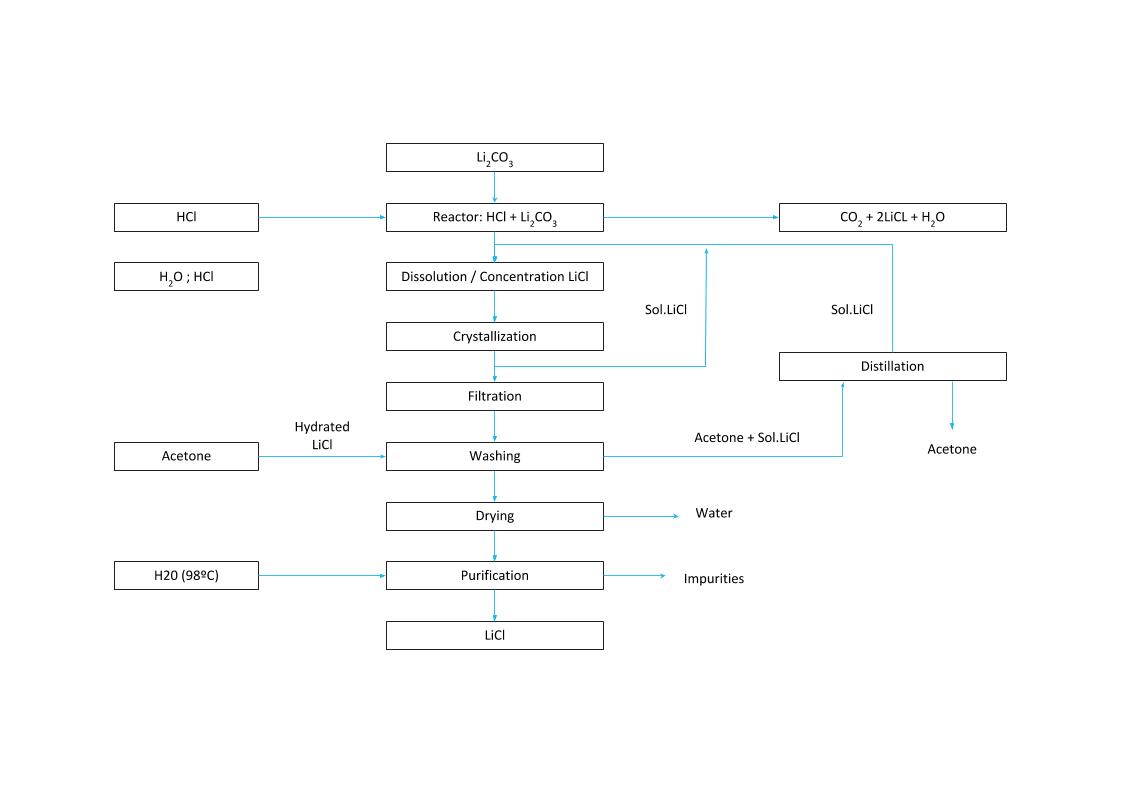
The process to obtain lithium chloride from carbonate or lithium hydroxide is achieved by reaction with hydrochloric acid:
Li2CO3 + 2HCl === 2LiCl + H20 + C02
LiOH·H2O + HCl === LiCl + 2 H2O
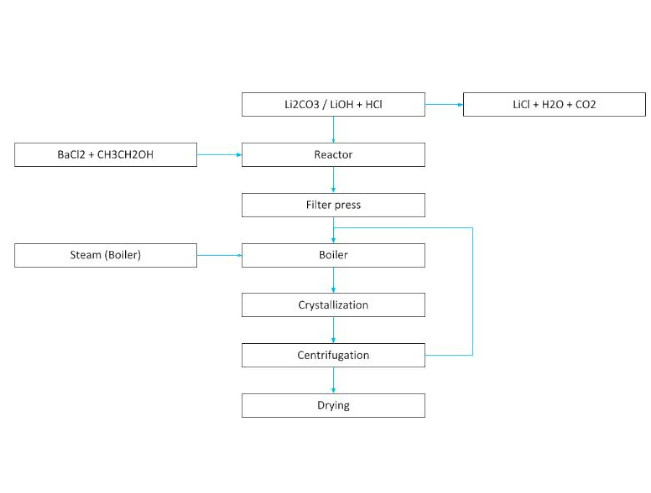
To remove the sulfate and calcium in the carbonate brine, oxalic acid and barium chloride need to be added to the reactor. Subsequently, the brine is filtered to remove its impurities.
The process then continues to the crystallization, centrifugation and drying stages. The crystallized lithium chloride is backwashed with cold water and subsequently sieved.
Metallic Li can be obtained from LiCl, which is useful in Li-Al alloys and in primary batteries (energy sources).
One of the advantages of this element is that it can be heated to 600ºC without decomposing. When heated to 800ºC in a hydrogen atmosphere, it partially decomposes into lithium oxide and carbon dioxide, a poorly soluble compound.
LiCl CRYSTALLIZATION PROCESS
Chemical process without natural evaporation
LITHIUM CRYSTALLIZATION PROCESS (2)
Example 2. Differential process of obtaining LiCl
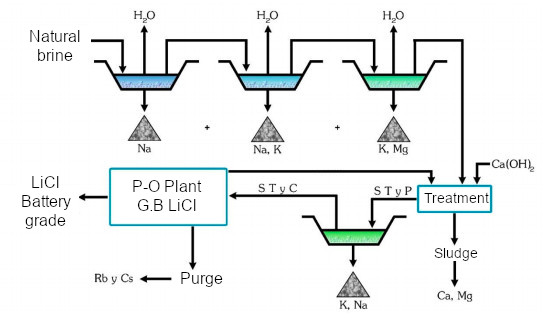
In a succession of sequential evaporation ponds as indicated in the diagram above, sodium, sodium and potassium, potassium and magnesium chlorides precipitate due to differential solubility.
Once an approximate concentration of 6% LiCl has been reached, lime is added to precipitate the magnesium, while boron can be removed as an isopropyl alcohol ester by solvent extraction with its recovery by distillation.
D. Galli described these processes in detail in the ADI company patent that corresponds to the exploitation of the Rincón salt flat in Salta.
Pond scheme in the evaporation process. Source: Dr D. Galli.
After adding lime, Mg, Ca and B are separated and the process can follow various alternatives depending on the lithium compound required: by adding Solvay soda, impure lithium carbonate is precipitated, which is then converted to lithium bicarbonate after addition of CO2, which gives battery grade Li2CO3 after filtration and heating.
Alternatively, high purity LiOH.H2O and LiCl can be obtained by electrodialysis of the concentrated lithium chloride solution. The company Simbol has developed a patented method for the purification of LiOH by LiCl electrodialysis to obtain battery grade.
As an alternative to the previous process, the brine can be treated in exchange resin columns that separate the contaminants before Solvay soda is added to obtain high purity lithium carbonate. It should be noted that the elution and regeneration of the columns leads to the formation of large volumes of liquid waste.
The soda-lime process precipitates Mg2+ and SO42– as Mg and Ca sulfate, which constitute the sludge contaminant. This can be used to consolidate paths in the development of the salt flat or as lithium material, as a strategic natural fireproof filler resource for buildings. Due to its negative effect on obtaining metallic lithium, boron must be removed by extraction with solvents such as isopropyl alcohol, forming esters.
Using the cal-soda method, technical grade Li2CO3 (> 99.5%) is obtained, which can be re-dissolved as soluble bicarbonate, LiHCO3, by bubbling CO2, filtering and increasing the temperature to remove CO2 and precipitating the battery-grade lithium carbonate with re-cycled CO2.
It is important to note that this process could be implemented as a fixation of atmospheric carbon dioxide with the consequent gain in green bonds. High purity lithium chloride can be formed by redissolving in isopropanol which is distilled to recover the solvent.
Also, lithium carbonate can be dissolved in HCl and treated on ion exchange columns to obtain high purity LiCl. Finally, metallic lithium is obtained by electrolysis of a molten eutectic mixture of KCl-LiCl at about 400°C under an argon atmosphere The use of solvents other than water can be expensive.
However, recovering them by distillation increases energy costs, so the impact on the final product cost must be carefully evaluated.
Salt flats high in Mg, such as Atacama and Uyuni, can be problematic, due to the flocculation of magnesium hydroxide during lime precipitation.
In these cases, magnesium should be removed initially by precipitation with Ca(OH)2 before the solar radiation evaporation concentration stage. In salt flats high in magnesium, the sulfate route is preferred to the chloride one.
Example 3. Selective lithium recovery (LiOH)
The selective recovery of lithium from brines with a content of less than 1% in the presence of high concentrations of other alkaline and alkaline earth ions is an industrial objective.
Evaporative processes are based on differential solubility of lithium salts in concentrated brine solutions which is called fractional recrystallization.
Alternatively, selective chemical and electrochemical processes have been designed for the recovery of high purity lithium chloride, lithium hydroxide or lithium carbonate that seek to reduce process times and reduce environmental impact due to water loss and the formation of environmentally harmful waste.
Recently, a rapid method based on the precipitation of low-soluble lithium phosphate, Li3PO4 (0.39g/l) has been proposed for treatment of brines with phosphoric acid. Then the insoluble lithium phosphate is treated with lime to form very insoluble hydroxyapatite and recover soluble lithium hydroxide.
3Li3PO4 + 5Ca(OH)2 -> Ca5(PO4)3OH + 9LiOH
Processes of extracting lithium from Argentine salt mines. In this process phosphoric acid is recovered by treatment of hydroxyapatite with sulfuric acid, with formation of hydrated calcium sulphate (gypsum) that has applications for construction:
Ca5(PO4)3OH + 5H2SO4 -> 5CaSO42H2O + H3PO4
This method has been patented by the Korean steel company Posco, which installed a pilot plant in Cachauri, Jujuy, in 2015. The method does not process brines through evaporation so it is significantly faster than evaporative methods.
However, because it uses phosphoric acid, which although recovered, can leave magnesium and calcium phosphate residues in the form of contaminating sludge.
Adsorption methods
Selective adsorption of lithium (300-1000 ppm) in brine and seawater (0.125 ppm) has been extensively studied using adsorbents such as MnO2, TiO2 and aluminum hydroxide. The uptake of lithium in these systems depends on the intercalation of lithium ions in non-stoichiometric networks of these oxides with a capacity of 3-35 mg/g that varies according to the type of adsorbent.
When extracting solutions rich in lithium ions such as brines (> 5 mg/L), uptakes of >20 mg/g can be achieved. In certain cases, other ions in the brine can co-insert, such as Mg, Na, K and Ca.
Manganese oxide has been studied as an adsorbent in various matrices such as MnOx ionic sieves with subsequent recovery of lithium by acid leaching to give, for example, Li0.15H0.76Mg0.40MnIII0.08MnIV1.59 O4.
The cubic spinel oxide structure -MnO2 can incorporate 38 mg/g to give LiMn2O4 by intercalation in the cubic lattice.
This alternative has been evaluated by Korean researchers in Uyuni (Bolivia). However, the stability of the oxide in leachate columns was not sufficient for an industrial scale process.
Acid treatment replaces the lithium ion with protons in the crystalline structure during elution, the mixed oxide dissolves and other ions such as Ca2+, Mg2+ are eluted.
Attention has been paid to rocks that can take up lithium in the Earth’s crust as model systems for the adsorption and absorption of lithium in their structures.
For example gibbsite, an aluminum hydroxide mineral, has been studied in detail for lithium uptake. Various companies have patented lithium recovery methods using various forms of amorphous aluminum hydroxide, including Dow Chemicals, Foote Mineral Company (FMC), Simbol Inc. and Posco.
The international mining corporation, FMC, through its subsidiary Minera del Altiplano, SA, with operations in Argentina in the Salar del Hombre Muerto, Catamarca, uses a proprietary technology method based on temperature-controlled ion exchange with zeolites, probably of the gibbsite type.
In these methods, lithium ions are extracted from concentrated brine containing LiCl after pre-concentration at 9 g/L, generally by solar evaporation. Liquid is then circulated through a polycrystalline hydrated aluminum hydroxide column supported in aggregate material until lithium saturation occurs.
In the second stage, LiCl from the ion exchanger is repetitively displaced with a concentrated solution of NaCl and finally a dilute solution of LiOH.
Ion exchange resins, such as Zeo-karb 225, Diaion SK and AG50WX8 with sulfonate groups and chelating agents have been used to capture lithium from synthetic brine.
Extraction with organic solvents of lithium entrapped with organic agents has also been proposed. In these cases, the costs of the resins, the energy involved in their regeneration and the solvents, as well as the possible environmental impact of the effluents, are critical.
Electrochemical methods
Electrochemical methods to extract lithium from brine have little impact on either water loss from evaporation or the environment, with chemical waste such as NaCl or MgSO4, and in turn are not excessively expensive.
Kanoh reported lithium ion intercalation on λ-MnO2 cathodes using an electrochemical cell with a platinum anode and studied the ion insertion/extraction kinetics of λ-MnO2/LiMn2O4 in contact with LiCl solutions.
The disadvantage of this cell is the reaction at the anode that modifies the pH of the brine by decomposing the water. La Mantia and collaborators used entropy cells to extract lithium using battery-type electrodes, a LiFePO4 cathode and Ag/AgCl anode, without pH changes in the brine but with a high cost of silver and dissolution in highly concentrated chloride solutions.
More recently, the same authors introduced a nickel hexacyanoferrate anode that exchanges cations as an alternative to the Ag/AgCl electrode. A similar electrochemical cell combining λ-MnO2 with a Ag anode was reported by Lee to extract lithium from artificial brine.
Kim, meanwhile, used the same manganese oxide cathode combined with a carbon capacitive electrode in a supercapacitor configuration.
These configurations were recently analyzed by Missoni. Similar methods, highly selective for lithium with respect to sodium, use an electrochemical process with a battery-type cathode of olivine structure, LiFePO4, coated with dopamine with I-/I3 -.
Hoshino proposed electrodialysis with an ionic liquid membrane but at a very low extraction rate.
Also deserving a mention is the method proposed by Liu, with two electrodes of LiFePO4 and FePO4 separated by a membrane permeable to anions for the extraction of lithium from brine.
The lithium ions produced in the LiFePO4 are combined with X- anions, increasing the concentration of LiX, while lithium ions are intercalated in the FePO4 electrode, decreasing the concentration of LiX in that compartment.
In the processes of extracting lithium from deposits in Argentine salt flats, Argentine researchers at Inquimae have developed an alternative method of extracting lithium from natural Puna brine, which was patented by Conicet.
This electrochemical method is fast, has a low environmental impact due to not adding chemical substances or producing waste, a low energy cost and is highly selective for the extraction of LiCl.
The proof of concept has been performed and the engineering for the development and scaling of the reactors is currently under development. The brine circulates through a non-membrane-divided electrochemical cell using a battery type lithium manganese oxide, Li1-XMn2O4 (LMO) (0 x 1) as cathode that selectively captures Li+ by intercalation in the solid, and the conductor polymer. polypyrrole (PPy), as an anode, that selectively captures Cl- ions by compensation of the charge after oxidizing this pseudo-capacitive electrode.
The brine is first exposed to the reduced Li1-xMn2O4 electrodes and oxidized PPy, with the LiCl being spontaneously captured with a release of energy.
After rinsing the electrodes, the brine is replaced by a dilute electrolyte and the polarity of the cell is reversed, thus recovering the LiCl in solution.
Under a potential difference of less than 1 V, the Li+ ions are intercalated in the Li1-XMn2O4 and the Cl- ions are adsorbed on the oxidized PPy.
The energy needed for the second process and the extraction and circulation pumps can be obtained from solar panels in the Puna region with solar radiation of over 2.600 kWh/m2 throughout the year; making it one of the best regions in the world to harvest solar energy.
The capital investment in solar panels with a 30-year lifespan was assessed at just $10 per ton of lithium chloride removed.
During the uptake of LiCl, only the Li+ ions are selectively intercalated in the manganese oxide in contact with the highly concentrated brine containing sodium, potassium and magnesium, for example.
The LiMn2O4 spinel is a stable phase with half the lithium content in the discharge from λ-MnO2 to Li2Mn2O4. The LiMn2O4 has the cubic spinel structure (space group Fd3m) and a unitary crystal cell containing 56 atoms: A packed oxygen ion structure at 32 sites with 16 Mn at octahedral (MnO6) sites and 6 lithium atoms at tetrahedral sites 8a.
The insertion and extraction of Li+ ions takes place by topotactic transition within the cubic structure, with isotropic expansion as revealed by the shift of reflections in X-ray diffractometry.
Using a chloride-selective electrode, lithium chloride can be highly selectively extracted from brine by adjusting the redox potential of the Mn(III)/Mn(IV) system in the crystal structure.
Due to the existence of two types of non-equivalent tetrahedral sites for the Li+ in the spinel, two oxidation-reduction processes are observed in this positive electrode material in batteries.
The process to extract lithium chloride from the brine is highly selective and efficient within the LiMn2O4/λ-MnO2 stoichiometry with high reproducibility for more than 200 charge and discharge cycles, low water consumption, and low energy consumption of 5 Wh/mol, based on charge, and 10 Wh/mol, based on the recovered lithium concentration.
No co-insertion of sodium or magnesium ions in manganese oxide has been observed by X-ray diffraction.
Detailed engineering and scaling of electrochemical reactors for the extraction of lithium from natural brine is currently being developed using this method [32].
The LiMn2O4 has a lithium uptake capacity of 38 mg/g; as it is the lightest metal, lithium can store a lot of charge per unit mass.
However, a lot of charge is required when it is recovered electrochemically: each 7 g of lithium requires a charge of 1 Faraday or 26.8 Ah. This has given rise to the “lithium paradox” for lithium extraction processes from deposits in Argentine salt flats; thus, careful design of the reactor with 3-D electrodes and a large specific area is essential.
Lithium recovery solutions
Our solutions to assist Lithium extraction from spodumene are:
- Solution 1. Concentration of the Li2SO4 solution
- Solution 2. Purification of Li2CO3
- Solution 3. Na2SO4 recovery as a byproduct during Li2CO3 production.
- Solution 4. LiOH production by reaction of Li2CO3 with lime
- Solution 5. LiOH production by electrodialysis
- Solution 6. LiCl production
- Solution 7. Water contaminated (tailings) treatment.
Solution 1. Concentration of the Li2SO4 solution
The sulfuric acid process for lithium extraction normally involves:
- Heating process at 1100ºC in a rotary kiln (α-spodumene is converted to β-spodumene)
- β-spodumene is grounded, mixed and roasted with H2SO4 at 250ºC.
- At this stage, Li2SO4 (soluble in water) is generated and an insoluble ore residue
- The excess of H2SO4 is neutralized with CaCO3. The kiln is then leached with water
- Filtration for impurities removal
- Lime addition to precipitate magnesium and soda ash to precipitate residual calcium
- pH adjustment with H2SO4, and Li2SO4 solution concentration by multiple-e ffect evaporation (triple effect) [1]. A highly concentrated lithium-containing solution is required
- Eventually, lithium can be precipitated as Li2CO3 by soda ash addition. Following crystallization, technical grade lithium carbonate is produced.
The utilisation of multiple-effect evaporation can assist the lithium concentration process before lithium carbonate precipitation
Solution 2 : Purification of Li2CO3
Technical-grade lithium carbonate is redissolved for impurities removal. By recrystallization, battery-grade lithium carbonate can be produced.
The utilization of crystallization technologies can facilitate battery-grade lithium carbonate production
Solution 3 : Na2SO4 recovery as a byproduct during lithium
Carbonate production:
- Na2CO3 is added to a concentrated Li2SO4 solution in order to precipitatelithium carbonate
- By evaporation and crystallization, lithium carbonate is obtained
- A residual solution containing sodium sulfate can be eventually concentrated in acrystallizer for Na2SO4 (by-product) recovery.
The utilisation of evaporation-crystallization technology can facilitate sodium sulfate recovery (by-product).
Solution 4. LiOH production by reaction of Li2CO3 with Ca(OH)2
Li2CO3 + Ca(OH)2 –> 2LiOH + CaCO3
A monohydrate LiOH can be produced by evaporation-crystallization.
The utilisation of crystallization technologies provided by Condorchem Envitech can assist during in LiOH production from Li2CO3.
Solution 5. LiOH production by electrodialysis
During electrodialysis (ED) treatment of a Li2SO4 solution, the cathode chamber is enriched with LiOH while the anode one is enriched in H2SO4.
However, other alkali metals like sodium and potassium will also be enriched in the cathode chamber. This could be solved by implementing a crystallization process following ED.
NaOH and KOH solubility is 10 times higher than LiOH solubility. Therefore, it is feasible to precipitate most LiOH while keeping NaOH and KOH in the solution.
The utilisation of crystallization technologies provided by Condorchem Envitech can assist during in LiOH production.
Solution 6. LiCl production
Lithium chloride can be produced by treatment of lithium carbonate with HCl.
Li2CO3 + 2HCI –> 2LiCI + H2CO3
The utilisation of crystallization technologies provided by Condorchem Envitech can facilitate the LiCl production process.
Solution 7. Tailings treatment
A ZLD solution is targeted. Opportunities for water and metals recovery can be available. Reduced effluent volume.
A combination of water and solid waste treatment technologies is required.
The utilisation of membrane and evaporation-based concentration technologies provided by Condorchem Envitech can facilitate a ZLD solution.
Bibliography
[1] O. A. Hougen, K.M. Watson, R. A. Ragatz, Principios de los Procesos Químicos. Parte
II Termodinámica, Editorial Reverté, Madrid, 1964.
[2] O. A. Hougen, K.M. Watson, R. A. Ragatz, Principios de los Procesos Químicos. Parte I
Balances de Materia y Energía, Editorial Reverté, Madrid, 1964.
[3] J. M. Smith, H. C. Van Ness, Introducción a la Termodinámica en Ingeniería Química,
3ª Edición, Editorial McGraw-Hill, 1982.CAP.1. INTRODUCCIÓN 9
[4] Roine, A., HSC Chemistry Software, Versión 5.11, Outokumpu Research Oy,
Información Servie P. O. Box 60 FIN-28101 PORI, Finland, 2005.
[5] L. David, Parkhurst, C. A. J. Appelo, User’s Guide to PHREEQC (version 2), U. S.
Geolical Survey Box 25046, MS 418, Denver, Colorado, 1999.
[6] gPROMS ModelBuilder version 2.3.1, Process Systems Enterprise Limited, 2004.
[7] K. S. Pitzer, J. Phys. Chem., 77(1973) 268-277.
[8] H. Renon, J. M. Prausnitz, AIChe J., 14 (1968) 135-144.
[9] C.F. Weber, Eng. Chem. Data, 39 (2000) 4422-4426.
[10] Y. Li, P. Song, S. Xia, S. Gao, CALPHAD, 24 (2000) 295-308.
[11] F. Farelo, C. Fernandes, A. Avelino, J. Chem. Eng. Data, 50(2005) 1470-1477.
[12] C. Monnin, M. Dubois, N. Papaiconomou, J. P. Simonin, J. Chem. Eng. Data, 47 (2002)
1331-1336.
[13] Chr. Christov, Chr. Balarew, S. Petrenko, Vl. Valyashko, Journal of Solution Chemistry,
23 (1994) 595-604.
[14] Z. Li, T. Deng, M. Liao, Fluid Phase Equilibria, 293 (2010) 42-46.
[15] Chr. Christov, Computer Coupling of Phase Diagrams and Thermochemistry, 36 (2012)
71-81.
[16] D. Zeng, Z. Wu, Y. Yao, H. Han, J. Solution Chem, 39 (2010) 1360-1376.
[17] J. M. Prausnitz, R. N. Lichtenthaler, E. G. Azevedo, Molecular Thermodynamic of
Fluid-Phase Equilibria, Prentice-Hall, Inc, Englewood Cliffs, NJ, 1998.
[18] D. A. Weingaertner, S. Lynn, D. N. Hanson, Ind. Eng. Chem. Res., 30 (1991) 490-501.
[19] T. A. Graber, H. Medina, H. R. Galleguillos, M. E. Taboada, J. Chem. Eng. Data, 52
(2007) 1262-1267.
[20] Y. T. Wu, D. Q. Lin, Z. Q. Zhu, L. H. Mei, Fluid Phase Equilibria, 124 (1996) 67-69.