Sections
- Introduction
- Manufacturing Process
- Main Applications of Cellophane
- Global Market of the Cellophane Industry
- Treatment of Effluents Derived from the Cellophane Manufacturing Process
- Summary
INTRODUCTION
Among the products manufactured by the paper industry, cellophane stands out for its properties that make it a clear candidate for replacing plastic packaging and bags derived from petroleum. Cellophane is one of the thinnest films derived from cellulose.
This polymer tends to be characterized by being transparent and flexible. Additionally, it is a highly resistant material for tensile work and easy to cut. It is made from the dissolution of hemp, cotton, or wood fibers. From this dissolution, a viscous solution is obtained, which undergoes an extrusion process and is subsequently bathed in an acid that converts it back into cellulose.
This process is very similar to the production of rayon fiber, although it differs in its extrusion process as cellophane passes through a slot while rayon passes through a hole.

Cellophane was invented by Swiss chemist Jacques E. Brandenberger in 1900. Inspired by seeing a wine spill on a restaurant tablecloth, he decided to create a fabric that could repel liquids instead of absorbing them.
His first step was to spray a waterproof layer onto the fabric, and he chose to treat it with viscose.
The resulting coated fabric was too rigid, but the clear film easily separated from the reinforcing fabric, and he abandoned his original idea when the possibilities of the new material became evident.
Brandenberger took ten years to perfect his film, with the main improvement over previous works with this type of film being the addition of glycerin to soften the material. By 1912, he had built a machine for the production of the film, which he called cellophane, from the words “cellulose” and “diaphanous” (transparent). This product was patented that same year.
Cellophane began to be used by an American candy company. This company used it as wrapping for its sweets. Over time, cellophane was marketed as a useful material within the food industry.
It was subsequently refined and adapted for various applications, such as Nitrocellulose, which provided resistance to external moisture, and other compounds that allowed for flexible and porous fibers, such as those used in sausage casings.
CHEMICAL STRUCTURE
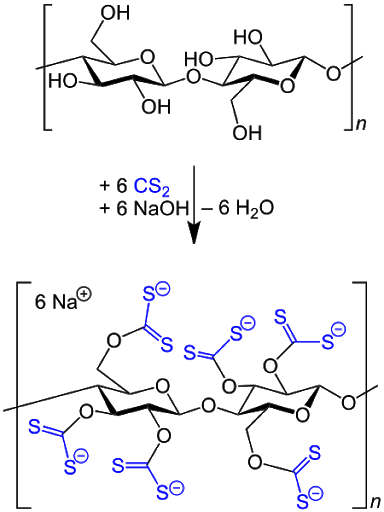
Cellulose is treated with carbon disulfide and alkali (sodium hydroxide) to produce viscose, which is then extruded through a slot and immersed in an acid bath that converts it back into cellulose.
Through a similar process, using a hole instead of a slot, a fiber called rayon is produced.
MANUFACTURING PROCESS
Cellophane or regenerated cellulose is a transparent plastic material made from cellulose by mixing cellulose xanthate with a diluted sodium hydroxide solution to form a viscous solution.
The cellophane film is transparent, colorless, non-toxic, and odorless, composed of regenerated cellulose, water, and a suitable humectant (plasticizer or softener), which is generally glycerol. Due to its low cost and a wide range of useful properties, cellophane is one of the most widely used films today.
Although it is not strictly considered a plastic material, cellophane is manufactured through an extrusion process in which a dissolved cellulose solution (viscose) is passed into an acid bath. This insolubilizes the solution and regenerates the cellulose.
Physical properties, such as tensile strength, elongation, softness, and rigidity, depend on the composition of this three-component system, which varies considerably within the following approximate limits: regenerated cellulose 60-85%, humectant 10-25%, and water 5-15%. The moisture content will vary additionally because the film is susceptible to changes in humidity in the atmosphere.
Cellophane is resistant and generally chemically inert, except to concentrated acids and alkalis. It also transmits a high percentage of ultraviolet rays. It is available in a variety of standard colors, can be modified to resist flames, and can be printed and decorated using various printing techniques.
The polluting effects of carbon disulfide and other byproducts from the viscose production process are toxic; however, cellophane itself is 100% biodegradable; and unlike plastic, whose recycling process is limited as it does not completely disappear from nature, cellophane completes this process through composting and returns to the earth in the form of fertilizer, closing the product cycle. A process that takes no more than five years and makes it a real alternative to traditional plastic.
MAIN APPLICATIONS OF CELLOPHANE
Cellophane is used as a general protective wrapping material. Due to its good electrical properties, it is used in the construction of wires and cables and other electrical products. It also serves as a separating, barrier, or release film in plastic molding and lamination. To make it moisture-proof, most cellophane film is coated with a lacquer made of nitrocellulose (pyroxylin), plasticizers, resins, and waxes. This coating can also give the cellophane film a heat-sealing property.
Additionally, thanks to its semipermeability, cellulose film is widely used as a membrane for dialysis and is the most popular material for making cigarette packs; its moisture permeability makes cellophane the perfect product for this application.
Among the most common uses and applications of cellophane are the following:
- It is used as wrapping for food, gifts, and floral arrangements.
- It is used for the production of adhesive tapes.
- In industrial applications, it is used for the production of semipermeable membranes, which are used for batteries.
- It can be applied as a coating.
- A derived product is used as a wrapping for sausages.
- The future holds an important place for it among substitutes for petroleum-derived polymers such as polyethylene or polypropylene.
But it is not only used in the food sector; it is also used in economic activities or businesses where it is necessary to wrap delicate objects gently, allowing the contents to be seen, such as in florists, jewelry stores, or gourmet bakeries. For all these reasons, it can be confidently stated that using cellophane in our professional or personal activities is an exercise in ecological and social responsibility.
THE GLOBAL MARKET OF THE CELLOPHANE INDUSTRY
The first commercial viscose production plants appeared in the early 20th century in Great Britain, the United States, France, Germany, and Russia.
Initially, viscose was marketed as filament until the 1930s when it began to be mixed with cotton and wool, creating new fabrics and options for the fashion industry.
The harmful effects of viscose production became increasingly evident, and by the end of the last century, many highly polluting and unprofitable plants were closed and moved to Asia, where there was cheaper labor and more lenient environmental regulations.
In the first decade of the 21st century, China quadrupled its viscose production capacity and now represents 66% of global production. India and Indonesia are the second and third largest producers worldwide. The industry also survived in Europe, where strong controls have led to the creation of closed-loop or circular systems so that the chemicals used do not escape into the environment or come into contact with workers.
The Viscose Market Today
According to information provided by Lenzing AG, global consumption of this fiber grew by 1.5% in 2016, reaching 99 million tons.
According to Changing Markets Foundation, the fiber is primarily used for the clothing industry (53%) but also in the home textiles industry (21%), industry (20%), and medical textiles (6%).
And by fiber, according to Lenzing AG, the product is distributed in the market as follows:
- Synthetic fibers from petroleum: 62.7% of the market
- Fibers from cellulose and protein:
- Cotton: 24.3%
- Cellulose fibers from wood: 6.6%
- Natural fibers: 5.3%
- Wool: 1.1%
If we observe the market share of production capacity worldwide by regions (2016), we see that China is the main producer of this material, followed by India (11.10%), Southeast Asia (10.36%), and Europe (10.03%).
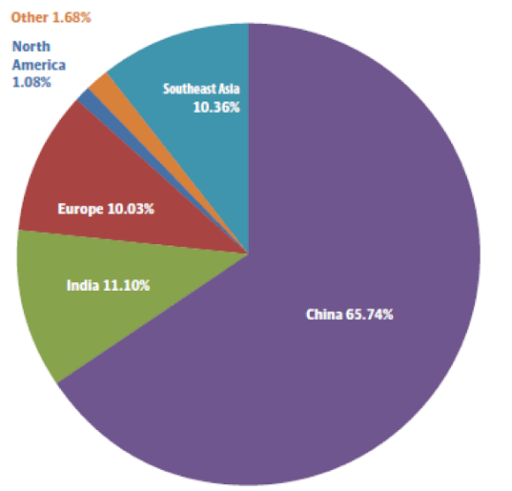
Source: Global Viscose Fibre Market Research Report.2017, May 2017, QYR Chemical & Materials
Many global manufacturers have opted to buy viscose generated in China and countries that can sell at a lower price at the expense of a significant environmental impact. The following diagram indicates some of these companies.
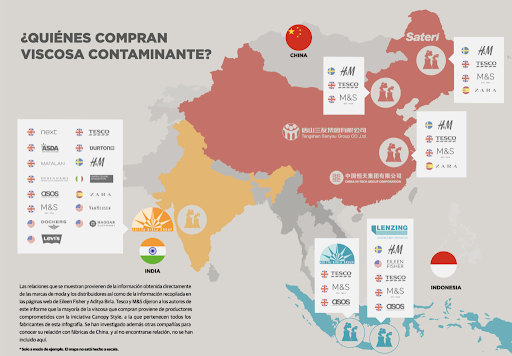
TREATMENT OF EFFLUENTS DERIVED FROM THE CELLOPHANE MANUFACTURING PROCESS
As we have discussed in this article, cellophane is a plant-based polymer that is 100% biodegradable, so its increasing use as a substitute for plastics is perfectly justified in order to reduce environmental pollution; however, the production process of viscose and the derivatives obtained from it generate highly polluting effluents that must be treated by sophisticated and costly facilities.
Biological Treatments
When the type of effluents allows it (relatively biodegradable), complex biological treatments are carried out, following a previous physicochemical treatment aimed at separating solids and neutralizing the discharges. An example of this is biological treatment in moving beds.
Moving Bed Technology
One of the technologies used for treating effluents from cellophane factories is a complex biological process that combines MBBR (Moving Bed Biofilm Reactor) and BAS (Biofilm Activated Sludge) processes with nutrient limitation, which has been successfully employed.
The BAS process consists of implementing a mixed system in which the influent pollutant load is treated by a combination of biomass adhered to a mobile support and suspended biomass. It combines a moving bed reactor at the front, followed by an activated sludge reactor.
A proper design of the process results in correct sizing in the activated sludge stage along with an MBBR biofilm reactor, capable of withstanding peaks of pollutant loads. The result is an effective and robust treatment suitable for any type of industrial wastewater.
The initial MBBR moving bed reactor is designed to remove the more easily biodegradable compounds, reducing the overall volume of the installation and improving the sedimentation properties of the sludge compared to those obtained in conventional activated sludge processes.
This first reactor also provides great stability against variations in influent load, dampening the peaks of load and the effects of any toxic or inhibitor, eliminating between 50-70% of the incoming BOD.
Additionally, this pretreatment increases the treatment capacity by 2 to 3 times compared to a conventional activated sludge process, while requiring a smaller volume.
The recirculation of sludge generates a mixed culture in the activated sludge reactor, where suspended biomass will combine with the biofilm generated in the previous moving bed reactor.
Likewise, the BAS process improves the characteristics of the activated sludge, making it more stable and with a sludge quality that is easier to dehydrate. The BAS process with nutrient limitation has an adjustment function, which reduces sludge production in cases where nutrient addition is required.
In the biofilm stage, the COD of the wastewater is transformed into polysaccharides, which in turn are used for the generation of new biomass in the activated sludge. The production and consumption of polysaccharides imply energy consumption for the bacteria, which limits their growth, resulting in reduced sludge production and thus savings in operational costs.
This system allows for reduced secondary sludge production and decreased nutrient requirements.
Excess sludge is concentrated through mechanical drying processes and the dosing of additives that facilitate its dryness, but in this case, they are difficult to compost as fertilizer, while vacuum evaporation technology does allow it, especially in cases where there is excess heat sources in the factory (hot water, steam, etc.).
Chemical Oxidation Treatments
It is also common to encounter discharges with very poorly biodegradable contaminations. These effluents include strongly acidic and alkaline discharges, as well as organic compounds that are difficult to biologically oxidize, so the organic load is considered as refractory COD. Treatment in these cases is costly, as it requires specific oxidation technologies.
After a pretreatment of solid separation with automatic screens and the neutralization and homogenization of effluents, a chemical oxidation treatment is carried out, which can be total or partial.
In partial oxidation, the aim is to break the bonds of complex organic molecules so that they can later be digested by bacteria in a subsequent biological treatment, while in total oxidation, a discharge is achieved that significantly reduces these organic compounds.
In the first case, the facilities are simpler, but the contact times are long, which imposes a high volume and large surfaces to install the treatment plants, which is not always feasible.
In the second case, the facilities require less space, but have a higher installation cost and energy consumption.
In some cases, oxidants based on chlorine were used, but the formation of organochlorines and THMs has rendered them obsolete.
Technologies that combine ozone with low and medium load UV radiation have also been tested, but even using catalysts, the results obtained have not been entirely satisfactory.
Vacuum evaporation is a technology applicable in cases where the volatile COD is low and, especially, if there is excess energy available in the factory, and in any case, it is applicable for concentrating the obtained sludges.
SUMMARY
Cellophane is a polymer generated from cellulose. It is highly biodegradable, which gives it an increasing environmental role compared to plastics. However, despite being a product perfectly compatible with food and possessing excellent physicochemical characteristics, its current manufacturing process requires reagents and treatments that produce highly polluting and complex effluents, to the point that the largest global production has shifted to Asian countries where environmental regulations are less stringent.
There is thus an important task in optimizing production processes and applicable purification technologies, as well as in regulating and controlling the origin of manufactured products and raw materials.
Bibliography and Internet References
- “Treatment of Industrial and Hazardous Discharges.” Welson L. Nemerow and Avigit Dasgupta – Ed. Díaz de Santos 1998.
- Metcalf-Eddy. 1994. Sanitary Engineering. Treatment, Disposal, and Reuse of Wastewater. Ed. Labor. Barcelona.
- https://www.navarra.es/NR/rdonlyres/454BE94F-2114-4831-BE6C-A617E2CA1369/90744/ VISCOFAN.pdf
- https://fashionunited.es/noticias/moda/la-viscosa-una-fibra-sostenible/2017070724133
“`