Sections
- The paper industry and the importance of water in its processes
- Paper manufacturing process
- Wastewater from the paper industry
- Effluent treatment
- Treatment of waste by vacuum evaporation
- Conclusion
The paper industry and the importance of water in its processes
Paper manufacturing is one of the main manufacturing industries worldwide, providing a wide range of essential products for our daily lives.
The paper production process generates large amounts of wastewater that, if not treated properly, can have a negative impact on the environment and public health.
Paper consists of a network of plant fibers with a high cellulose content that have been treated through various processes, laid out on a screen, and finally dried.
These fibers can come from different plants and trees, but the most commonly used source is coniferous wood, due to the high length and strength of its fibers. One-third of all wood processed in the world is intended for paper and pulp production.
Paper manufacturing consumes various resources:
- Raw Material (Approx. 2.5 tons of wood / ton of paper)
- Chemicals (NaOH, sulfites, oxidants, etc.)
- Water and Energy (approx. 10 – 15 m³/ton of paper)
In the paper manufacturing process, water is used as:
- Medium for disintegrating raw materials
- Transport of fibers
- Formation of paper
Paper manufacturing process
Separation of cellulose
The process begins with the separation of cellulose from the rest of the substances (lignin, oils, resins, etc.), which accounts for 50% by weight.

Two systems can be used for extracting cellulose fibers:
- Mechanical pulp: grinding the wood. The quality of the obtained pulp is lower, but less liquid waste is produced.
- Chemical pulp: subjecting wood chips to a chemical treatment to solubilize lignin so that cellulose fibers are released.
The chemicals used can be:
- Alkaline products: This method generates highly polluting black liquor effluents, which are treated to recover sodium sulfide and caustic soda.
- Sulfites: In this system, some of the chemicals used can also be recovered, such as sulfuric acid.
Chemicals that cannot be recovered are lost with the residual effluents, along with cellulose residues that have not been retained and contribute a high COD to the effluent.
Pulp bleaching
The remaining lignin alongside the cellulose fibers gives color to the pulp, especially in the case of mechanical pulp.
To obtain white pulp, the pulp must undergo a bleaching process, which can be carried out in different ways:
- Hydrogen Peroxide: although it does not eliminate lignin, it does remove color.
- Chlorine gas or chlorine dioxide: This process is much less environmentally sustainable as it generates a large amount of organochlorine compounds, including dioxins and furans.
- Ozone: This option does not generate by-products and has thus replaced the use of chlorine.
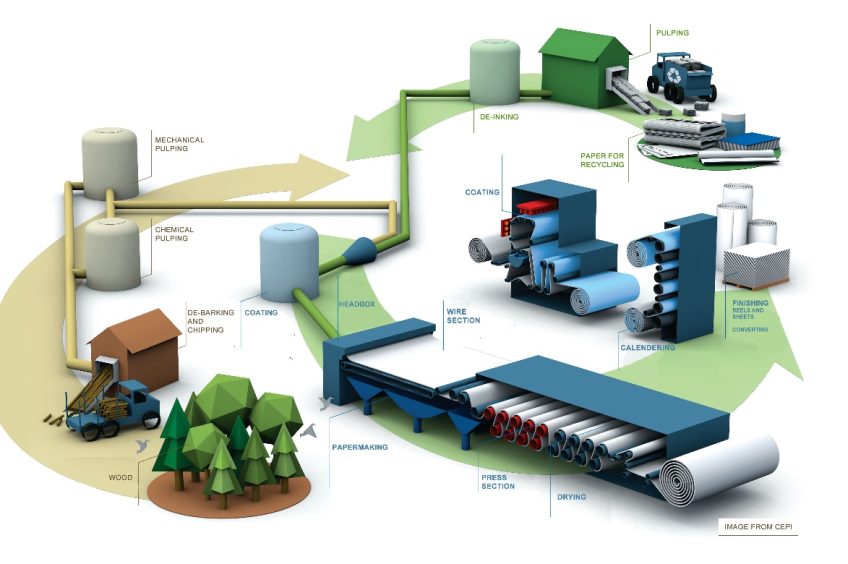
The mixture of different types of wet pulp with filling substances (calcium carbonate, kaolin, titanium dioxide, etc.) and other additives (aluminum sulfate, dyes, starch, latex, etc.) is evenly spread on a metal support and dried, resulting in paper.
For the production of writing or printing paper, the paper surface is subsequently smoothed using mechanical means.
Wastewater from the paper industry
During paper production, a large volume of water is consumed, which must also be of high quality. The wastewater from paper manufacturing mainly comes from three sources:
- The residual liquid produced during pulp treatment (black liquor).
- The white water from paper machines, produced during the manufacturing process.
- The wash, screening, and bleaching discharges of the pulp. These discharges are loaded with pollutants that must be separated for reuse or disposal.
Approximately 1 to 15 m3 of water is needed per ton of wood used to make paper, depending on whether water reuse occurs.
The generated effluents contain high pollution due to more than 250 different compounds.
Some are of natural origin and come from wood (lignin, tannins, etc.), while others are synthetic and are incorporated into the effluent during the manufacturing and bleaching processes of cellulose pulps, such as phenols, dioxins, and furans.
As mentioned, the pulp and paper manufacturing process generates wastewater with complex characteristics due to the great diversity of substances found in the residual effluent. The main pollutants include:
- Organic material: cellulose fibers, lignin, and other wood residues.
- Suspended solids: remnants of fibers and particles.
- Toxic compounds: chlorine derivatives in bleaching, such as dioxins and furans.
- Nutrients: nitrogen and phosphorus.
Chemical Oxygen Demand (COD) and Biological Oxygen Demand are indicators of the amount of organic matter that requires oxygen for decomposition.
The treatment of this wastewater aims to reduce or eliminate these pollutants to comply with environmental regulations and avoid damage to the ecosystem.
Below is an average analysis of the discharges found at different stages of the wastewater treatment process in a paper industry:
| Sampling point | SS (mg/l) | COD (mg/l) | BOD5 (mg/l) |
|---|---|---|---|
| Effluent to be treated | 1200 | 1200 | |
| Effluent after Physicochemical treatment | 50 | 240 | 120 |
| Final effluent | ≤20 | ≤150 | ≤25 |
It is important to note that the production of pulp and paper requires good quality water to achieve a good product.
Depending on the location of the factory, it may be necessary to consume water from municipal supply networks, which should be avoided as much as possible.
Therefore, in addition to recycling treated wastewater from the factory itself, it has also been opted, at times, to use recycled water from nearby wastewater treatment plants.

Effluent treatment
There are two options for treating the wastewater produced by paper industries, depending on whether part of their discharges is reused or not:
Open cycle: Treatment of water without reuse
The simplest option is to properly treat the effluents and discharge the treated effluent into the environment in compliance with regulations and avoiding any environmental impact.
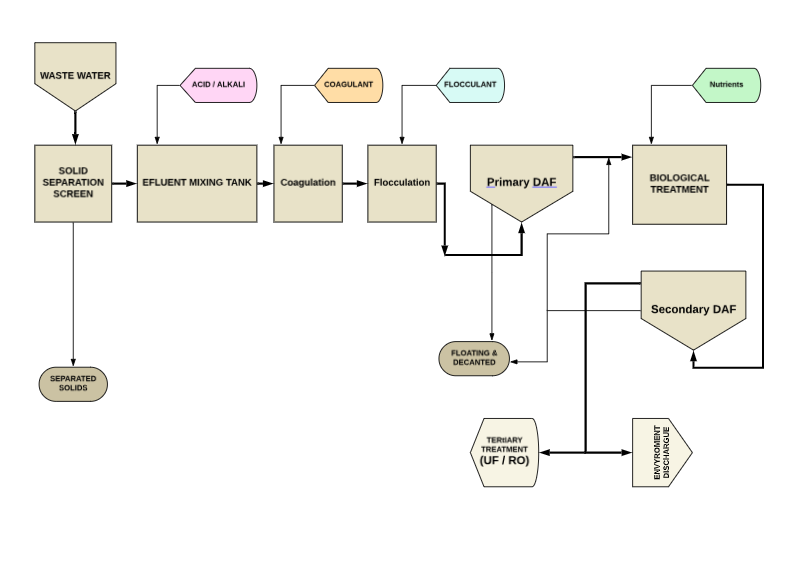
A satisfactory treatment of the effluents would include the following stages:
- Homogenization and pH neutralization
- Coagulation-flocculation followed by sedimentation or DAF flotation
- Removal of organic matter through a biological process (anaerobic or aerobic), or through advanced oxidation (with ozone, Fenton, or photo-Fenton).
Closed cycle: Treatment of water with reuse
There is a more sustainable and often more economical alternative, which consists of treating the effluents to recover water for reuse.
Since the production of pulp and paper requires water at numerous stages of the production process, incorporating wastewater treatment technologies into the production chain allows the same water stream to be continuously reused in the same stage of the process or sent and utilized in another stage.
With a closed cycle, a dual objective is achieved:
- Minimization of water consumption
- Minimization of liquid waste produced
Thanks to this system, the environmental impact of the process as a whole is reduced.
To obtain water of sufficient quality for reuse, a more thorough treatment is required than that described in the Open Cycle. The main stages of this treatment are as follows:
- Homogenization, pH neutralization, and sedimentation or DAF flotation: these processes separate colloids and larger suspended solids
- Advanced oxidation (preferably ozonation): this stage destroys large organic molecules that may be refractory in a subsequent biological process.
- Anaerobic/aerobic biological treatment: reduces the content of dissolved organic matter in the liquid. The anaerobic process produces biogas that can be used as fuel for energy generation.
- Clarification of the effluent through sedimentation or DAF flotation
- Filtration of the digestion effluent with ultrafiltration (UF): first through sand filters and then with ultrafiltration membranes.
- Reverse osmosis treatment: the ultrafiltered water is subjected to a reverse osmosis process, after which water of the necessary quality for reuse in the paper manufacturing process is obtained.
- Vacuum evaporation: the remaining residues and rejects can be treated by vacuum evaporation to minimize their volume. The water recovered in the evaporation can also be reused, while the concentrate, reduced to its minimum expression, must be managed as waste.
- Incineration: The generated sludge, along with plant residues such as tree bark, sawdust, etc., can be burned in a boiler that provides energy to the industry processes.
At this point, it is important to remember that if the wastewater treatment plant includes an anaerobic reactor, the biogas (mainly methane) produced from biological digestion should be added as fuel.
The sum of these energy sources serves to meet the energy requirements of the evaporator and the overall process of the factory.
Treatment of waste by vacuum evaporators
Vacuum evaporators allow for the reduction of liquid waste volume and the recovery of valuable resources.
This process takes advantage of the decrease in the boiling point of liquids under reduced pressure conditions, allowing evaporation at lower temperatures than normal.
Principles and benefits of vacuum evaporation
Vacuum evaporation is based on the inverse relationship between pressure and the boiling temperature of a liquid.
By reducing the pressure in a closed system, liquids begin to evaporate at considerably lower temperatures.
In the paper industry, liquid effluents usually contain water, organic and inorganic pollutants, and chemical compounds used in manufacturing and treatment processes.
Vacuum evaporation allows for a significant reduction in the amount of liquid waste while facilitating the recovery of chemicals, such as bleaching agents or adhesives, that can be reused in the production chain, reducing raw material costs.
Additionally, vacuum evaporators recover a high percentage of high-quality water, which can be incorporated into the production process due to its high purity.
In summary, vacuum evaporation is a high-performance technology with a clear application in the paper industry, which is increasingly aligned with sustainability and resource optimization trends.
Conclusion
The paper manufacturing industries and their derivatives consume significant amounts of water for their production processes. Additionally, they generate highly contaminated discharges due to the separation of products contained in the raw material, as well as the reagents used for the various treatments in the process.
Given the growing demand for paper and the associated water consumption, it is necessary to improve the processes for treating the produced wastewater, while also modifying the industrial processes followed in paper manufacturing.
The goal that industries are setting is the recovery of treated discharges with the aim of achieving the so-called “zero discharge,” to minimize environmental impact by reducing water consumption and the generation of waste; for this, high-performance water treatment technologies must be used.
Vacuum evaporation represents an innovative and effective solution for the treatment of wastewater in the paper and pulp industry. Its ability to minimize waste volume and facilitate water recovery makes it an attractive option for companies committed to sustainability and operational efficiency.
Bibliography and inquiries
– DAF technology applied in the paper manufacturing industry for the recycling of wastewater – News – Wuxi Gongyuan Environmental Equipment&Technology Co., Ltd (gyeclarifier.com)
– PPT – Paper PowerPoint Presentation – ID:2632688 (slideserve.com)
– What is the environmental impact of paper consumption – (ecologiaverde.com)