Sections
- Purge Streams from Thermal Power Plants
- Contaminants Present in Desulfurization Purges
- Treatment of Desulfurization Discharges
- Conclusions
Purge Streams from Thermal Power Plants
Thermal power plants generate electricity from the combustion of fossil fuels such as coal, oil, and natural gas. They are one of the main sources of energy supply but also among the most polluting.

The environmental impact caused by thermal power plants manifests in various ways.
- The increase in CO₂ concentrations, one of the main greenhouse gases, induces climate change, causing extreme weather events.
- Atmospheric pollutants such as SO₂ and NOx can lead to the formation of acid rain, which harms soils, water, and vegetation, compromising the health of ecosystems and the biodiversity that depends on them.
To comply with sulfur emission limits established by environmental regulations, thermal power plants use a process called desulfurization.
The gases from coal combustion in the boiler, once passed through electro-filters, are pushed by fans towards a gas-to-gas heater where their temperature is increased.
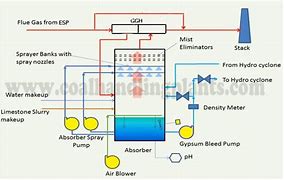
Once the required temperature is reached, they enter a *scrubber*, where the lime slurry reacts with SO₂ and uses the O₂ from the air to promote oxidation to CaSO₄, according to the reaction:
CaCO3 + SO2 + 2 H2O + ½ O2 → CaSO4 + 2 H2O + CO2
The resulting gases are sent to a chimney to avoid condensation and are discharged outside at a temperature above the sulfur precipitation point. Thanks to this desulfurization process, SO₂ is reduced by 95%.
As a result of the process, a gypsum slurry remains at the bottom of the absorber, which, once dried, is transported to a waste manager. Alternatively, the possibility of using this residue in some applications, such as soil remediation, is being evaluated.
Most of the filtrate water is reused in the same process, and only a small part (purge) is sent to the effluent treatment plant.
The treatment of purges from the *scrubber* mainly consists of a physicochemical process followed by a vacuum evaporator, in which brackish water is treated. After evaporation, high-quality distilled water is obtained and recycled to the absorber, while the concentrate represents less than 5-10% by volume of the treated water. This process can achieve ZERO DISCHARGE.
Contaminants Present in Desulfurization Purges
Desulfurization purges from thermal power plants contain several contaminants that must be properly treated to avoid environmental damage:
- Sulfur Dioxide (SO₂). It is the main contaminant removed in the desulfurization process.
- Nitrogen Oxides (NOx). These compounds are also generated during combustion and must be controlled.
- Suspended Particles. Including ashes and dust generated during combustion.
- Volatile Organic Compounds (VOCs). They may be present in small amounts and must be eliminated.
- Heavy Metals. Such as mercury and lead, which may be present in combustion gases.
The following analysis can be considered typical of a desulfurization purge from a thermal power plant:
| Parameter | Unit | Concentration |
|---|---|---|
| pH | – | 4-7 |
| Suspended Solids | mg/l | 10,000 |
| SO4=-2 | mg/l | 15,100 |
| SO3 -2 | mg/l | 200 |
| F- | mg/l | 50 |
| P | mg/l | 5,100 |
| NH4+ | mg/l | 406 |
| NO3- | mg/l | 100 |
| NO2- | mg/l | 100 |
| Al+3 | mg/l | 10 |
| Cd+2 | mg/l | 4 |
| Cr+6 | mg/l | 30 |
| Cu+ | mg/l | 20 |
| Fe+3 | mg/l | 1 |
| Pb+2 | mg/l | 100 |
| Hg + | mg/l | 0.9 |
| Ni+3 | mg/l | 30 |
| Zn+2 | mg/l | 20 |
| Cl- | mg/l | 5,000 |
| Mg+2 | mg/l | 3,700 |
| P | mg/l | 500 |
| Oils and Fats | mg/l | 1 |
| COD5 | mg O2/l | 800 |
| TOC | mg O2/l | 270 |
| Max. Temperature | ºC | 47 |
Suspended solids are composed of 89% CaSO4·2 H2O + 2% CaCO3 + remaining 9% of CaF2 and others). As can be seen, the effluent has a high content of SS, SO4-2, NH3, and different metals, among which the most toxic are: Cd+2, Pb+2, Cr+6, and Hg+1. Also, in this case, a high concentration of NH3 is observed.
Treatment of Desulfurization Discharges
The treatment of wastewater produced in desulfurization purges from thermal power plants is a complex process involving several stages to remove different contaminants and comply with environmental regulations.
The design of the treatment plant may vary depending on various factors, but these are the most commonly used technologies:
- Physicochemical Treatment: This is the first step and consists of removing suspended solids and other inorganic contaminants through processes such as sedimentation and coagulation-flocculation.
- Biological Reactor (when toxicity allows): In this stage, microorganisms are used to break down dissolved organic matter in the water. This method is effective in reducing compounds such as nitrogen and phosphorus.
- Advanced Treatment: Also known as tertiary treatment, this stage focuses on removing residual contaminants not eliminated in previous stages. It may include processes such as membrane filtration, adsorption, and advanced oxidation.
- Vacuum Evaporation: This method is used to separate water from contaminants by evaporation, obtaining a high-quality condensate that can be recycled, for example, in the desulfurization process itself.
- Heavy Metals Treatment: In this stage, heavy metals such as mercury and lead are removed by techniques like chemical precipitation and adsorption.
Each of these methods has its advantages and disadvantages, and the choice of the appropriate treatment depends on the specific characteristics of the wastewater and treatment goals.
As an example, let’s see what treatment would be most suitable to purify a desulfurization purge with an analysis like the one shown in the previous table.
The treatment line would basically consist of:
- Solid Separation. Purges contain solids of considerable size that must be separated before the purification process; screens and grates with appropriate filtration openings (approx. 1 – 3 mm) are commonly used. Solids are automatically removed by a surface scraper placed over the screen.
- Homogenization and Oxidation Tank. Inside, the effluent is mixed and homogenized by mechanical means and air supplied from blowers. This way, SO₃²⁻ is oxidized to SO₄²⁻ and NO₂⁻ to NO₃⁻, and oxidation of organic and inorganic materials sensitive to it is facilitated.
- Precipitate Sedimentation. From the homogenization tank, the effluent flows by gravity to an adjacent clarifier-thickener, from which a clarified liquid exits on one side and suspended solids (mostly CaSO₄) settle on the other. The separated solids undergo dehydration to be sent to a waste landfill.
- Physicochemical Treatment. This is a complex process carried out in several stages to separate remaining suspended solids as well as contaminant metals.
- Vacuum Evaporation. The next step is to separate soluble salts (Cl⁻, NO₃⁻, SO₄²⁻, etc.) and traces of metals (Cr, Pb, etc.) that may remain in the effluent. For this, the effluent is sent to a vacuum evaporator where a concentrate of salts and metals and a contaminant-free condensate are obtained.
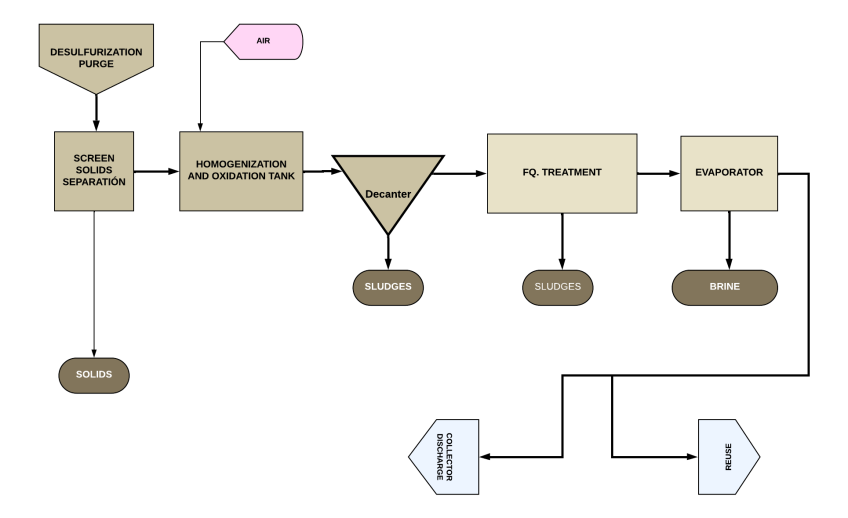
Vacuum evaporation is based on reducing pressure to lower the boiling point of water. This allows water to evaporate at lower temperatures, separating it from contaminants in the concentrate.
Vacuum evaporation is an efficient process to obtain high-quality water and reduce the amount of waste.
Advantages
- Energy Efficiency: This process requires less energy compared to other methods due to the low operating temperatures (in our case, desulfurization purges are usually hot, approximately 45ºC).
- Water Quality: A high-quality condensate is produced that can be reused in the desulfurization process.
- Waste Reduction: Minimizes the amount of solid waste and allows more efficient handling of contaminants.
Considerations
- Corrosion: System materials must be corrosion-resistant due to the chemical nature of the purges.
- Scaling: The formation of deposits must be controlled to maintain process efficiency.
- Excess NH₃ Removal: It is common for discharges from desulfurization processes to have high ammonia (NH₃) concentrations. To remove it, stripping towers are usually installed, operating by air injection. This air exceeds the partial pressure of ammonia, facilitating its transfer from the liquid phase to the gas phase, where it is subsequently diluted in the outgoing air flow.

Conclusions
Thermal power plants emit a wide variety of contaminants contained in the gases produced during combustion, such as carbon dioxide (CO₂), nitrogen oxides (NOx), sulfur dioxide (SO₂), and suspended particles.
For gas treatment, separation systems using *scrubbers* are employed, which absorb a significant portion of the contaminants. The purge from this circuit must be treated in an appropriate facility due to its high contaminant concentrations.
The most common process for treating desulfurization purges consists of:
- Pretreatment: Solid separation
- Homogenization and Oxidation: Discharges are mixed in a tank and contaminant compounds are oxidized with air.
- Clarification: Using a clarifier-thickener.
- Physicochemical Treatment: Composed of several stages according to the contaminants present (SS, metals, etc.).
- Vacuum Evaporation: The effluent free of suspended solids and metals contains high salinity, which can be treated in a vacuum evaporator. Vacuum evaporation allows obtaining a high-quality condensate and a concentrated residue, with reduced energy costs and environmental impact. The obtained condensate will have sufficient quality to be used in various processes and services, amortizing the investment cost in a short time.
- Sludge Treatment: Both sludges obtained by mechanical dehydration means, such as filter presses, and those from the evaporator itself, must be sent to special landfills due to their toxic nature.
- When NH₃ concentration is high, excess must be removed. The most economical and simple system would be a stripping tower, equipped with ring packing, through which an air stream is passed countercurrent to the liquid circulation.
Bibliography and references:
https://ecologiadigital.bio/ – Pollutant emissions from thermal power plants and their environmental impact