Air pollution poses a serious threat to health in most areas of the planet. According to an assessment of the morbidity burden due to environmental pollution conducted by the WHO, more than 7 million premature deaths attributable to the effects of urban pollution occur each year. Furthermore, it is not a problem exclusive to the more developed countries, as more than half of this burden falls on the population of developing countries.
Nitrogen oxides are not the only contributors to air pollution, but they are among the most significant pollutants.
Nitrogen oxides are two different nitrogen gases: nitric oxide (NO) and nitrogen dioxide (NO2). The term NOX refers to the combination of these gases due to their mutual interconversion capabilities in the presence of oxygen. Although formally, the general term nitrogen oxides encompasses the following compounds:
- NO
- NO2
- N2O2
- N2O4
- N2O
- N2O3
- N2O5
- NO3 (the latter being unstable)
Although a large part of NOX is of natural origin, a significant fraction of NOX is due to anthropogenic processes. The most important artificial sources correspond to transportation (70%) and industry (25%). The industrial processes that generate NOX in greater quantities are those dedicated to energy production, the combustion of coal, oil, or natural gas, and the processes of electroplating and metal etching. NO and NO2 are formed in processes where, in the presence of nitrogen and oxygen from the air, temperatures exceed 1200 ºC.
Nitrogen oxides all share the common characteristic of being pollutant gases, so their emissions have a significant impact on the environment. The main effects they cause are:
- The destruction of stratospheric ozone
- Contribution to the greenhouse effect
- The production of acid rain
- The generation of photochemical smog
For all these reasons, it is essential, first and foremost, to minimize their production. Subsequently, it is necessary to eliminate the nitrogen oxides that could not be prevented from being generated. The goal of minimizing their generation can be achieved by following three different strategies:
- Reducing the operating temperature
- Reducing the residence time of the gases, especially nitrogen, in the combustion zone, where high temperatures exist
- Decreasing the oxygen-fuel ratio. By reducing the excess oxygen, the generation of NOX is significantly decreased
However, it is impossible to completely avoid the generation of nitrogen oxides, and to comply with increasingly stringent regulations, techniques must be employed to eliminate the generated NOX. The most commonly used techniques for this purpose are:
Absorption through chemical reaction
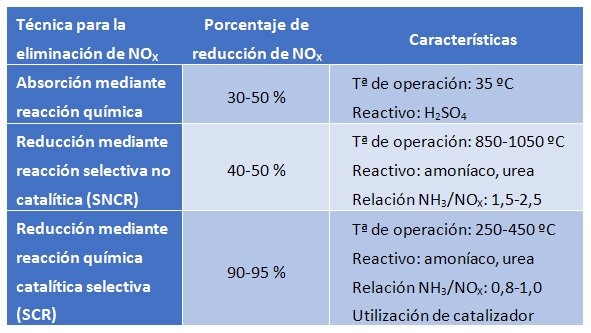
This technique involves the absorption of NOX through a chemical reaction in the liquid phase. The most commonly used reagent for absorption is sulfuric acid. It reacts with nitrogen oxides to form the species HSO4NO (nitrosylsulfuric acid), which remains in the liquid phase. Under high pressure (2 atm) and low temperature (35 ºC), the NOX is absorbed in the liquid phase. Conversely, the process can be reversed at high temperature (180ºC) and low pressure (0.5 atm); under these conditions, the nitrogen molecule (now nitric acid due to the presence of water) is separated from the sulfuric acid, which can be reused.
This process has the disadvantage that corrosive and hazardous chemical reagents must be handled, and physical space is required to accommodate the process. The achieved efficiencies are not high, so the technique is recommended for low NOX loads.
Reduction through non-catalytic selective reaction (SNCR)
This technique allows for the reduction of nitrogen oxide emissions by converting them into nitrogen gas via a non-catalytic chemical reaction. To carry out this conversion without the presence of any catalyst, it is necessary to raise the temperature within the range of 850-1100 ºC. The operating temperature directly depends on the reducing agent used, with ammonia or urea being the most commonly used.
This technique is often used in small industrial boilers, as costs escalate in larger installations when operating in this temperature range. The SNCR equipment does not require a large space and is easy to install and operate. However, the reduction efficiency achieved is moderate, making it a valid technique for cases where nitrogen oxide emissions are low.
Reduction through selective catalytic chemical reaction (SCR)
This technique is based on a catalytic process in which nitrogen oxides are selectively reduced in the presence of a catalyst while the reducing agent (ammonia or urea) is oxidized to nitrogen gas. The fact that the reaction occurs on the surface of the catalyst makes it possible for the necessary temperature to be within the range of 250-450 ºC. The operating temperature will ultimately depend on several factors, with the catalyst used being one of the key parameters.
The reducing agent can practically be an aqueous solution of ammonia, liquefied ammonia, or an aqueous solution of urea. Among these, the use of liquefied ammonia is the most economical option, resulting in lower operating costs. However, on the other hand, handling liquefied ammonia is much more complex due to its characteristics than handling an aqueous solution of ammonia or urea. The use, storage, and transport of liquefied ammonia are subject to the Directive 96/82/EC (Seveso II Directive) and must be used following a strict safety protocol due to the risks associated with its highly corrosive and explosive nature in the presence of oxygen.
In terms of operation, the higher the NH3/NOX ratio fed, the greater the efficiency achieved. However, this will also increase the amount of unreacted ammonia that is wasted in the gas stream. This loss of unreacted ammonia must be minimized, as it reacts in the presence of water with SO3 to produce ammonium bisulfate (NH4HSO4), which is corrosive and causes fouling of the facilities. The key to optimal operation is to feed ammonia in such a way that good performance is achieved while minimizing the amount of unreacted ammonia.
The choice of catalyst is crucial in the process, as it influences key parameters such as operating temperature and the extent of the reaction. There are four different materials used as catalysts:
- Metal oxides (vanadium, tungsten, molybdenum, or chromium) based on titanium dioxide (TiO2)
- Zeolites
- Iron oxides coated with a thin layer of iron phosphate
- Activated carbon
The choice of catalyst also directly affects operating costs, as not all have the same properties, costs, and lifespans.
The main advantages of SCR technology are based on the high NOX removal efficiency, as well as the fact that NOX is converted into nitrogen gas without producing any byproducts or waste.
Thus, the emission of nitrogen oxides must be controlled as it is strictly regulated by current legislation. The first step in controlling them is to minimize the production of these gases. Production that cannot be prevented must be properly treated before releasing the remaining gases into the atmosphere. For the elimination of NOX, the most efficient technique is selective catalytic chemical reaction reduction (SCR).
Urea
As mentioned, urea is used as a reducing agent to eliminate nitrogen oxides (NOx) through SCR and SNCR; this illustration* shows the appearance of a urea molecule. Urea is also used as an additive in combustion engine vehicles to neutralize their emissions as much as possible.
*(credit 3dchem.com)