Sections
Introduction
The chemical elements known as Rare Earths, or Lanthanides, are found in the period corresponding to Lanthanum. They are commonly expressed as rare earth oxides under the designation ETR or REE, Rare Earth Elements. This group of elements consists of 15 elements with very similar chemical properties but differing in their physical properties, a circumstance that complicates their separation.
This sequence of elements exhibits, due to its electronic configuration, the lanthanide contraction. This fact represents the gradual decrease in atomic size as the atomic number increases. The atomic number of these elements varies from number 57 (La) to 71 (Lu), and this group includes Sc (21) and Y (39) (Puche, Cascales, Porcher, and Maestro) (Gambogi and Curier, 2010).
The electronic configuration of these elements indicates that their most stable oxidation state is trivalent, although +2 (Eu, Yb, Sm) and +4 (Ce, Pr, Tb) states can also be stabilized. Due to their electronic distribution, they exhibit peculiar magnetic and optical properties that are highlighted, for example, in the case of Neodymium (high-power magnets).
Rare earths correspond to 17 chemical elements, among which are the 15 lanthanides (lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, and lutetium), in addition to scandium and yttrium, which are included due to their physicochemical similarities with the lanthanides (mainly their electronic configuration, ionization potentials, highly electropositive character, and similarity in their ionic radius (+3)), making rare earth elements highly interchangeable among themselves in a large number of minerals (through a metal displacement reaction).
Rare earths are classified into three groups: light rare earth elements (LREEs), heavy rare earth elements (HREEs), and the middle rare earth group (MREEs), which encompasses from samarium to gadolinium.
Source Minerals
There are about 200 minerals in nature that contain rare earths in their composition. Typically, associations of different elements occur in the same mineral due to their similar chemical properties. Cerium is the rare earth element found in the highest proportion in the Earth’s crust (greater than copper), while Neodymium and Lanthanum are more abundant than Nickel, Lead, and Cobalt.
Currently, the main ores of rare earths are Monazite [(Ce, La, Th) PO4], Xenotime [(YPO4)3], and Loparite (Na,Ce,Sr)(Ce,Th)(Ti,Nb)2O6 as a lower-grade ore (Puche, Cascales, Porcher, and Maestro).
Monazite is a phosphate mineral, with the formula [(,,,ℎ)4], which corresponds to the second most important ore of LREEs with reserves in Australia, Brazil, China, India, Sri Lanka, and the United States. Deposits containing monazite often contain thorium, a radioactive element, which leads many countries to discard it as a source of rare earths due to the potential environmental damage.
The concentrations of rare earth elements and thorium in monazite mineral vary depending on the deposit, with an average of 70% rare earths (mainly cerium, neodymium, and lanthanum) and 12% thorium (uranium percentages can also be found, which, like thorium, is a radioactive element).
Monazite is a rare earth and thorium orthophosphate (RTh)PO4. It is the most abundant rare earth mineral and constitutes a byproduct of ilmenite that accompanies zircon.
Bastnäsite is a fluorocarbonate (RFCO3) that contains rare earths, especially Ce, Nd, Eu, and La. The most important reserves are found in Mongolia and California.
This fluorocarbonate mineral with the formula [(,)3(,)] is found in deposits in China and the United States, corresponding to the largest reserves of light rare earths (mainly lanthanum, cerium, and neodymium) in the world. They contain very low concentrations of thorium, and their rare earth content is approximately 70%.
Xenotime is a rare earth and yttrium orthophosphate, with a high content of cerium and thorium. The most important deposit of this mineral is located in Guangdong (China), although its uranium and thorium content and the associated radioactivity limit its exploitation. Other less relevant deposits are found in California (USA), Malaysia, and Indonesia.
Euxenite is a mineral with the formula [(,,,,ℎ)(,,)26]. It is usually found as tantaloniobate (minerals where Ta and Nb form the compound) of titanium, rare earths, thorium, and uranium. Deposits exist in Idaho (USA), Norway, and Madagascar.
Loparite is a niobium-titanate of rare earth located in the Kola Peninsula, Russia, with concentrates around 32% in rare earth oxides, mainly cerium.
Xenotime is the main mineral of HREEs in the world, specifically of yttrium. It contains an average of 67% rare earth elements, and the main deposits have a xenotime grade of 0.5-5%. There are deposits of this mineral in California (USA), Malaysia, and Indonesia.
Allanite is a mineral with the chemical formula [(,,)(22+)(//4) ∙ 27]. It is a mineral of the epidote group (a silicate mineral with a general formula of 23(4)3(,), where A and B can be replaced by different elements), present in three forms that contain cerium, lanthanum, and yttrium.
It is found in igneous, metamorphic, and hydrothermal environments. The percentage of radioactive elements (thorium and uranium) can vary from traces of the element up to 3%, while its concentration of rare earth elements averages around 5%.
Due to the presence of radioactive elements and its low concentration of rare earth elements, it is not extracted as a primary mineral in mining operations.
Finally, it is worth highlighting a certain type of clay rich in rare earths as an important reservoir. These specific clays originate from the weathering of common igneous rock containing rare earths, which can lead to leaching of these rocks and subsequent absorption on the surface of clayey aluminosilicate minerals (kaolinite, illite, and smectite), enriching them with rare earths and making them viable for economic extraction.
Although the concentrations of rare earth elements are usually low (average 0.3%) compared to the minerals described above, the processing of this type of deposit is less complex, making it economically competitive.
These types of deposits exist in southern China and Kazakhstan. Although clay reserves constitute only 2.9% of the reserves in China, they represent 35% of its rare earth exports.
Specifically, in China (Xungu, Longnam), there are reserves of more than 10MTm expressed as RO (rare earth oxides). These surface deposits in China are relatively easy to extract and highly profitable.
Rare earth ions are adsorbed by ion exchange in the crystal networks of the clays. Rare earth elements are recovered from the leaching of the clays with saline solutions. 50% of the world’s rare earth reserves are found in China.
The industrial procedures currently in use for their recovery are aimed at obtaining ETR concentrates from the indicated minerals. With the exception of bastnäsite, many ETR concentrates are obtained as by-products or results of pyrometallurgical or hydrometallurgical processes (Orrego, 1998; Orrego, 2000; Vega, 2000; Hedrick, 1999; Dwivedi, 1982; Harrah, 1967; Sunur et al., 1985; Alarcon, 1998; Lapido, 1994; Sundaram, 1987; Swanina and Nair, 1989; The Humphreys Investment Co, 1970).
Technological Properties
Globally, rare earths are elements that, thanks to their physical and chemical properties, have allowed their use in a series of cutting-edge products. First, the permanent magnet industry is becoming increasingly important in medical technologies (nuclear magnetic resonance techniques, PET), electronic technologies, mobility technologies (railway), hybrid technologies, etc.
Initially, permanent magnets were made from steels, ferritic metal alloys with low remanence and coercive fields. Rare earths offer high coercive field values and magnetic moments that exceed those of ferritic magnets.
Rare earths have their weak point in paramagnetism at room temperature. However, this point was overcome by the Sm2Co17 alloys and especially the more novel Nd2Fe34B, with magnetic energy values of 450KJ/m3. Likewise, GdTbFeCo alloys have been discovered to be highly efficient in memory systems.
The technological use of rare earths in the construction of optical materials is another current field of influence. Specifically, it is the construction of tricolor lamps in which the emitted light is a combination of three wavelengths: 450nm; Ba Mg2O16 Al27 Eu2+/556nm; (Ce/Tb)Mg Al11 O19/610nm; Y2O3Eu3+. These luminescent systems reproduce sunlight and achieve very high luminous efficiency.
In this same line, the use of rare earths in the technology of RX intensifier screens has been classical. Calcium tungstate (CaWO4) has traditionally been used.
These luminescent materials aim to reduce the patient’s radiation exposure time and achieve correct resolution. These materials are being replaced with the goal of improving this efficiency. Gd2O2ScTb+, LaOBrTm+, and YTaO.
Likewise, TV screens, computer monitors, and mobile screens reproduce color from chromophore groups doped with lanthanides. The red color, for example, is reproduced by yttrium oxysulfide doped with europium (Y2O3:Eu3+). Another application field of rare earths is in lasers, especially high-efficiency neodymium lasers with monochromaticity, coherence, and directionality. (Sáez, Cascales, Porcher, Maestro; 2000).
Extraction Process
Monazite
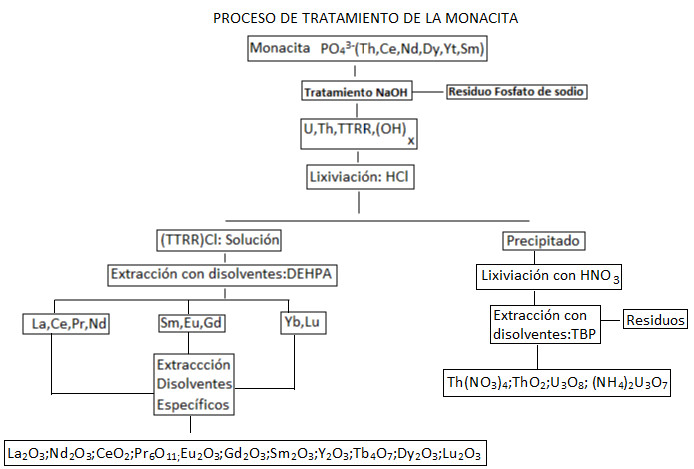
One of the most important rare earth-bearing minerals will be taken as a reference: monazite.
Starting from the monazite concentrate, it is treated with sulfuric acid at a temperature of 200ºC, with the aim of leaching the lanthanide elements that are part of the concentrate.
In the process, impurities such as uranium, thorium, certain phosphates, and sulfates are also dissolved, allowing for the secondary concentrate of this radioactive material. The solution will be slowly neutralized.
First, a treatment with ammonium hydroxide is carried out. At pH values of 1, thorium sulfate precipitates in its insoluble form. Along with thorium sulfate, a small portion of rare earths precipitates. At a pH value of 2.3, most of the rare earths precipitate.
Finally, at a pH of 6, uranium precipitates as hydroxyuranate. The precipitates corresponding to pH values of 1 and 6 are treated with tributyl phosphate to separate uranium from rare earths and improve the yield of the process. At this point, a rare earth concentrate is obtained.
By adding sodium sulfate, the precipitation of light rare earths (REE) occurs, yielding;
2(4)3 ∙ 24 ∙ 2
Yttrium, heavy rare earths, and radioactive elements remain in solution. The precipitate, once separated, is treated with sodium hydroxide to form a rare earth concentrate. This is dried at 120ºC.
The precipitate is redissolved in nitric acid, and thorium and cerium are selectively precipitated by adding ammonium hydroxide at a pH of 2.8.
Subsequently, after removing this first precipitate, ammonium hydroxide is continuously added, precipitating the rest of the rare earths as hydroxides. The heavy rare earth elements in solution can be separated from other radioactive elements and concentrated using an extractant such as tributyl phosphate.
To recover thorium and rare earths from the solution, the acidic solution is treated with sodium oxalate, precipitating thorium and rare earths at pH 1.5, while uranium remains in solution. The oxalate precipitate is treated with sodium hydroxide, precipitating the rare earths.
This precipitate is calcined and treated with nitric acid to redissolve and purify the extraction of rare earths. This acidic solution is treated with tributyl phosphate, yielding an organic extract with thorium and cerium and a solution with rare earths.
The extract is subjected to a purification stage with sodium nitrite, obtaining cerium nitrate in the aqueous phase and thorium nitrate in the organic phase.
Clays
The largest volume of rare earths extracted comes from clay deposits in China. The richness in rare earths is not high, but the ease of extraction favors its exploitation. This ease is based on the ionic form with which lanthanide ions interact with the silicate structure of the clay.
Rare earths are found in cationic form (+3), easily extractable. The extraction process is based on the use of concentrated solutions of monovalent cations (Na2SO44)2SO44Cl).
In the interaction, an ion exchange occurs between the monovalent ions and the lanthanide ions. The rare earths pass into solution as sulfates and/or chlorides.
This solution is treated with sodium oxalate so that insoluble oxalates of the lanthanides are formed, precipitating in the solution. This oxalate precipitate is calcined at 900ºC to obtain the oxides. This oxide concentrate recovers between 80-90% of the rare earths present in the original clay.
In Madagascar, seawater is used to leach the clays and subsequently precipitate the rare earths with oxalates.
Separation and Purification Processes
The technological use of these materials requires a high degree of purity in their obtaining. This has led to the development of new extraction systems that are more effective than traditional ones.
Rare earths can be leached using nitrates, chlorides, and sulfates. Once a rare earth concentrate is obtained, a separation and purification process must be initiated.
Selective Oxidation
First, a selective oxidation can be performed by oxidizing cerium, praseodymium, and terbium from a (+3) state to a (+4) state. In the case of praseodymium, if oxidation occurs under high oxygen pressure, it can approach the composition PrO2.
Praseodymium and terbium are not stable in aqueous solution when oxidized to their tetravalent state, making it relatively easy to precipitate them from a solution of rare earth hydroxides (formed by dissolving the oxide mixture with a potassium hydroxide solution), using potassium chlorate (KClO3) as the oxidizing agent.
Cerium is the most abundant rare earth element and, therefore, the one with the lowest commercial value. It can be oxidized to its tetravalent state by heating the oxide mixture to 650 ºC in air or by drying rare earth hydroxides in air at 120-130 ºC.
In aqueous solutions where the elements are dissolved as hydroxides, cerium can be oxidized by chlorination or electrolysis. Cerium can also be oxidized in solution by injecting ozone as the oxidizing agent. To recover oxidized cerium (tetravalent state), the mixture of rare earth elements can be selectively dissolved in diluted acid where cerium (IV) oxide has low solubility.
The black oxide dissolves in acid with the release of oxygen to give green solutions or green salts that have applications in the ceramics industry.
Elements such as samarium, europium, and ytterbium are less abundant than cesium; for this reason, they must be concentrated before being chemically treated for their recovery. Europium (III) can be reduced to europium (II) using a mercury cathode. The recovery of samarium can be performed from lithium amalgams.
Supercritical Extraction Method
Lanthanum, neodymium, samarium, europium, gadolinium, dysprosium, and holmium carbonates can be obtained from a rare earth extract by treating an aqueous suspension of the oxides with CO2 at a temperature equal to or greater than 31ºC and at a pressure of 71.2 atm.
Under these conditions, the carbonates Pr (III), Er(III), Yb(III), and Tb(III) do not form or do so with very low yield. This separation technique has also been used in the separation of lanthanides in the (III) oxidation state from those in the (IV) state.
The operating conditions are 40ºC and 100 atm, forming after one hour of reaction the carbonates of lanthanum, neodymium, samarium, europium, holmium, promethium, and thulium in the (III) oxidation state. In this way, a carbonated precipitate is obtained, which can be separated and treated with hydrochloric acid. This solution can be treated with solvents or ion exchange resins.
Solvent Extraction
Starting from a rare earth concentrate obtained from the leaching stage, the solvent extraction stage is fed. In this stage, heavy rare earths (terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, and yttrium), medium (samarium, europium, and gadolinium), and light (lanthanum, cerium, praseodymium, and neodymium) are separated.
Subsequently, through extraction with specific solvents, each specific rare earth oxide will be separated. Finally, purifications will be carried out using ion exchange techniques or chromatographic techniques. The main extractive agents in the field of rare earths are;
- Carboxylic Acids. These are affordable acids with moderate prices, despite the drawback of their solubility in water. Among the most commonly used carboxylic acids are versatic acid 10, naphthenic acids, and 2-bromodecanoic acid, all diluted in xylene. The solubility of naphthenic acid varies depending on the pH. At a pH of 4, the solubility is 0.09 g/l and varies up to 0.9 g/l at a pH of 6.5. The solubility of versatic acid ranges from 0.7g/l to 0.25g/l. The 2-bromodecanoic acid works at more acidic values. The steric effect of the acid molecule influences the extraction of lanthanides and is related to the atomic number of the metallic element.
- Alkyl Phosphoric Acids. The most used acid is DEHPA (di-2-ethylhexyl phosphoric acid). This acid is used diluted in kerosene. For example, HCl 0.1M using a solution of DEHPA 0.2M in kerosene. It is observed that the extraction of these elements improves in the order La < Ce < Nd < Sm < Eu < Gd < Tb < Dy = Y < Ho < Er < Tm < Yb < Lu, decreasing the distribution coefficient value with increasing temperature. Another type of alkyl phosphoric acid used is EHEHPA (2-ethylhexyl-2-ethylhexyl phosphoric acid), also diluted in kerosene. A formulation used is HCl 0.1M and the concentration of the extraction agent in kerosene 0.2M. In this system, the extraction of the metal increases with the atomic number, although it is lower compared to the DEHPA system. The use of phosphonic acids and/or phosphinic acids improves the separation between adjacent lanthanides.
- Hydroxylamines. Specifically, acidic agents with the ability to form chelates. These extractants have been commonly used in the treatment of Cu, but their utility in the treatment of rare earths is expanding. For example, SME 529 (currently LIX 84) has been used to study the extraction equilibrium of Ce(III) and La(III) in a sodium chloride medium, with the extraction agent diluted in n-heptane. LIX 70 has also been used for the extraction of these two rare earths. In this case, kerosene and n-heptane were tested as diluents for the organic phase, with the aqueous medium being NaCl. Both cerium and lanthanum are quantitatively extracted, although cerium can be extracted at lower pH values.
The type of amine and the aqueous medium decisively influence the extraction of these elements. Primary amines (RNH2) extract these metals from a sulfate medium, while tertiary amines (R3N) extract them from a nitrate medium.
In general, light rare earths are preferentially extracted with these basic extraction agents.
- TBP (Tributyl Phosphate) and DBBP (Di-n-butyl-n-butyl-phosphonate). Rare earths are extracted from different aqueous media. With them, europium and samarium are obtained. Their mode of action is based on the reaction: Ln3+aq + 3NO3– + 3Lorg < => Ln(NO3)3L3(org)
- Crown Ethers. They have been used for the extraction of lanthanides. (sim-dibenzo-16-crown-5-oxyacetic acid) in a 80:20 chloroform – heptanol solution. In the case of crown ethers, the extraction of various rare earths depends very specifically on the pH corresponding to the aqueous medium, and thus, Lu3+ is quantitatively extracted at a pH of 6.7, while La3+, Pr3+, Sm3+, Eu3+, Tb3+, Er3+, and Yb3+ do so almost quantitatively (> 98%) at a pH of 6.5. The stoichiometry of the extracted complex is 1:2 (metal: extraction agent). Calixarenes are (1,n)-cyclophanes that present a cavity formed by phenyl groups acting as bridges, allowing the introduction of substituents into the organic skeleton. This structure has led to these compounds being studied as reagents for the extraction of some trivalent lanthanides.
- Lanthanum, neodymium, europium, erbium, and ytterbium.
- Synergistic mixtures. Synergistic mixtures are replacing traditional single-extractant extraction systems. Synergistic mixtures, which are the combination of two or more extracting agents that increase the effectiveness of extraction and enhance the specificity of the separation operation, are becoming particularly important. For example, a phosphonic acid such as PC-88 A (2-ethylhexylphosphonic acid) dissolved in n-heptane along with DTPA (diethylamine-pentaacetic acid) has been studied for the extraction of lanthanides.
The separation factors between Y/Ho/Er are greater when adding DTPA, while the stoichiometry of the extracted species is represented by LnR3-3HR.
Conclusions
Rare earths were, a few years ago, simply the end of the periodic table that was not reached due to lack of time or simply ignorance. Nowadays, their technological properties and industrial applications have placed these minerals in the status of critical minerals for many governments.
In Executive Order 13817, dated December 20, 2017, the U.S. Government recognizes the vulnerability that external dependence on 35 minerals classified as critical minerals represents for a technological system, 17 of which are rare earths.
They are characterized by having high electrical conductivity and magnetic properties that make them optimal for manufacturing batteries, mobile phones, electric vehicle batteries, LED lighting systems, wind turbines, laser systems, satellite technologies, defense systems, etc.
These applications demand purity in the obtained metals and effective treatments of the concentrates. New methods such as the application of crown ethers, the use of synergistic mixtures like combinations of alkylphosphoric acids, or the application of quaternary ammonium salts will facilitate the optimization of the enrichment process.
Bibliography
F.J. Alguacil and F. Rodríguez, CSIC. Process for the separation of rare earths. Rev. Metal Madrid, 33 (3), 1997
Bautista, R.G. Mineral Proc. Extractive Met. Rev., 8, 1992: 175-182.
Bautista, R.G. and Jackson, N. Rare Earths, Resources, Science, Technology and Applications. TMS. Warrendale (USA), 1992.
Habashi, F. Rare Earths’90. Proc. Int. Symp. on Processing of Rare Metals. Osaka (Japan), 1990: 47-52.
Patricio Javier Avendaño Corvalán, Thesis “EVALUATION OF TECHNICAL-ENVIRONMENTAL FEASIBILITY OF A RARE EARTH EXTRACTION PLANT IN CHILE” Faculty of Chile, 2017
R. Sáez Puche, C. Cascales, P. Porcher, P. Maestro. “Rare Earths: Advanced Materials”. Faculty of Chemical Sciences, Complutense University of Madrid. CSIC. CNRS. Chemical Research. 2000