Sections
- What is electrocoagulation?
- Application in wastewater treatment
- Comparison with physicochemical coagulation/flocculation treatment
- Conclusions
- Bibliography and references
What is electrocoagulation?
Electrocoagulation is an effective and versatile water treatment technique, as it allows the removal of a wide range of contaminants from different types of water, including industrial wastewater, surface water, and groundwater.
The process consists of destabilizing the contaminants present in the water, whether suspended, emulsified, or dissolved, through the action of low-voltage direct electric current and metallic electrodes, usually aluminum/iron.
Electrocoagulation equipment is compact and operates continuously, using a reactor in which metallic electrodes are arranged inside to facilitate the flow of electric current.
In this process, a high concentration of cations is generated that neutralize the electrical charges of the colloids present in the water, leading to the formation of complex metal hydroxides. These compounds act as coagulant agents, forming flocs that incorporate contaminants thanks to their adsorption capacity. The flocs rise to the surface due to turbulence and the decrease in their apparent density caused by gases generated during the reaction.

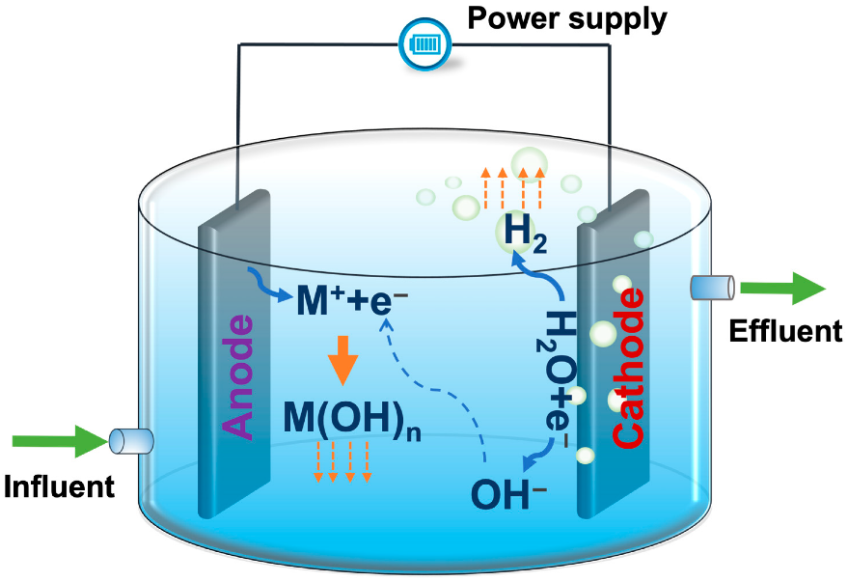
The chemical reactions that take place in the electrocoagulation process are as follows:
- Anode: M⁺ + e⁻
- Cathode: H₂O + e⁻
- Hydroxide: M(OH)ₙ ↓ + OH⁻
H2 gas is released, which rises at the cathode.
In the case of an Fe electrode, it would be:
- Anode: Fe → Fe²⁺ + 2e⁻
- Cathode: 2H₂O + 2e⁻ → H₂(g) + 2OH⁻
- Hydroxide: Fe²⁺ + 2OH⁻ → Fe(OH)₂ ↓
The following diagram shows the reactions involved in the process:
A wastewater treatment by electrocoagulation consists of the following stages:
- Neutralization of the electrical charges of the colloids, facilitated by the metallic cations generated at the anode.
- Adsorption of contaminants onto the metal hydroxide flocs formed *in situ*, which act as coagulant agents.
- Precipitation of contaminants along with corrosion products of the electrodes, mainly iron or aluminum, depending on the anode material.
- Separation by flotation, promoted by the drag of the flocs through microbubbles of hydrogen and oxygen generated at the cathode and anode, respectively.
- Oxidation and/or reduction of dissolved substances, thanks to the redox reactions occurring in the electrochemical environment.
On the other hand, chemical oxidation occurs, allowing the transformation of metals and contaminants into non-toxic species and significantly degrading COD/BOD.
One of the main benefits of electrocoagulation is its ability to remove contaminants that are difficult to treat with other methods. For example, it can be very effective in removing colloids, emulsions, heavy metals, and microorganisms. Additionally, it is a technology that maintains consistent performance over time.
When designing an electrocoagulation installation, it is essential to consider the following basic factors:
- Type of electrode: aluminum, iron, or alloys.
- Electrode configuration: monopolar, bipolar, in series or parallel.
- Distance between electrodes: influences electrical resistance.
- Current density: ranges between 5 and 50 mA/cm².
- Initial pH: affects the formation of active metallic species.
- Water conductivity: may require addition of electrolytes (NaCl, Na₂SO₄).
- Retention time is another factor that varies case by case.
Another notable benefit of electrocoagulation is its ability to break down complex organic compounds present in water, which is difficult to achieve with conventional water treatment methods.
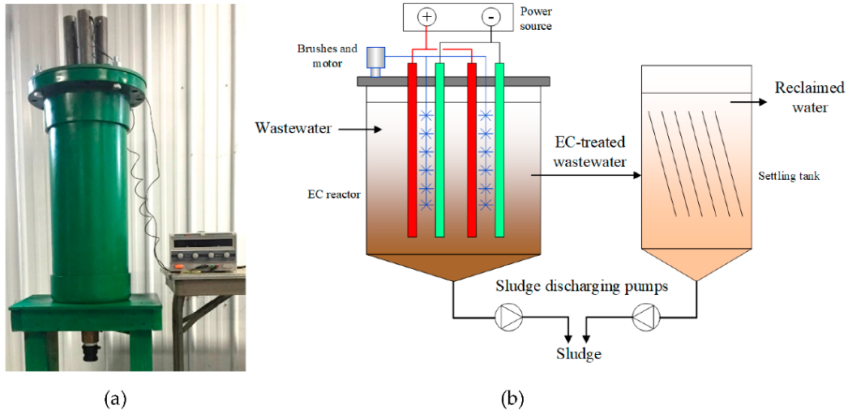
The components of a basic electrocoagulation system are:
- Electrochemical reactor with submerged electrodes.
- Direct current power supply, with voltage and current control.
- Agitation system to keep the solution homogeneous.
- Solid-liquid separator, which can be a sedimenter, filter, or flotation unit to remove the flocs.
Electrocoagulation is a technology in constant evolution, incorporating significant advances in the following areas:
- Use of coated inert electrodes to extend service life.
- Hybrid systems: EC + dissolved air flotation (DAF), EC + advanced oxidation.
- Automation with pH, turbidity, and conductivity sensors.
- Integration into decentralized or modular treatment systems.
Application of electrocoagulation in wastewater treatment
As indicated in the previous section, electrocoagulation is an effective process for treating wastewater containing colloidal and/or biological contamination.
Electrocoagulation uses electricity to remove contaminants. This process involves introducing electrodes into the wastewater, which release positive ions when an electric current is applied. These ions bind to contaminants present in the water, forming larger aggregates that can be easily separated from the water.
The main advantage of electrocoagulation is its effectiveness in removing a wide range of contaminants, including bacteria, viruses, heavy metals, and organic compounds.
Another important benefit is its low operating cost, especially when compared to other water treatment methods. This is achieved thanks to its low energy consumption, absence of coagulant and flocculant reagents, and the fact that no toxic secondary sludge is produced.
In summary, the main advantages of electrocoagulation for wastewater management are:
- No external addition of coagulants and flocculants required.
- Generation of efficient flocs.
- Low volume of sludge produced.
- Capability to treat complex effluents.
- Modular, automatable, and energy-efficient process.
Limitations include:
- Corrosion and wear of electrodes (periodic replacement required).
- Efficiency dependent on pH and conductivity.
- Electrode passivation.
Electrocoagulation is successfully applied in various sectors such as:
| Sector | Contaminants removed |
|---|---|
| Textile industry | Dyes, surfactants, COD, suspended solids |
| Tannery | Chromium, sulfates, solids, organic matter |
| Food industry | Fats, oils, solids, organic load |
| Urban wastewater | Phosphates, nitrogen, microorganisms |
| Electroplating | Heavy metals (Cu, Zn, Ni, Cr), cyanides |
| Pulp and paper industry | Organic matter, solids, color |
Comparison with physicochemical coagulation/flocculation treatment
Electrocoagulation stands out as an efficient, economical, and sustainable process, as it does not require the addition of external chemical coagulants or flocculants, thus minimizing environmental impact and reducing treatment costs. Additionally, the resulting sludge is less voluminous, more stable, and easier to handle than in other coagulation processes.
This process is especially useful for removing substances such as oils, fats, heavy metals, dyes, and microorganisms, making electrocoagulation a benchmark technology for wastewater purification.
The following table presents a comparison with the conventional chemical coagulation/flocculation process:
| Technique | Energy consumption | Reagent consumption | Sludge production | Space required | Sludge type | Maintenance |
|---|---|---|---|---|---|---|
| Electrocoagulation | Moderate | None | Low | Low | Stable | Complex |
| Coagulation/flocculation | Low | High | High | Medium | Unstable | Simple |
Despite its advantages, electrocoagulation is not free from challenges. In particular, energy consumption and electrode management may require special attention.
However, technological advances are helping to overcome these drawbacks, making electrocoagulation an increasingly viable and attractive option for wastewater treatment.
Conclusions
Electrocoagulation is a simple process that requires relatively simple equipment. The generated flocs contain little surface water, are resistant to acidic media, and exhibit good stability, facilitating their separation by filtration.
Moreover, it is a low-cost technology that demands a moderate initial investment.
It is a promising alternative for treating wastewater with contaminants that are difficult to remove by conventional methods. Its effectiveness, versatility, and sustainability make it an attractive option for multiple industrial and urban applications.
However, to achieve optimal implementation, it is essential to properly select operational parameters and ensure periodic system maintenance.
In summary, electrocoagulation is a versatile and effective water treatment method, capable of addressing a wide range of contaminants and significantly improving the quality of treated water.
Bibliography and references
1- Electrocoagulation of Wastewater: Innovative Solution for a Cleaner World | Water Institute
2- Techno-Economic Analysis of Electrocoagulation on Water Reclamation and Bacterial/Viral Indicator Reductions of a High-Strength Organic Wastewater—Anaerobic Digestion Effluent