Definition of reverse osmosis
Reverse Osmosis (RO) process consists of generating an aqueous solution with a low salt content from another aqueous solution that has a high salt content, via a water-permeable membrane.
It is the technology used to produce desalinated water from seawater. Just as in MF and UF, the driving force for achieving the separation of the salt is caused by a difference in transmembrane pressure.
Nevertheless, in RO, the separation process is due to the different solubility and diffusivity in the membrane of the aqueous solution components. The operating values for the transmembrane pressure difference and solution concentration are 7 – 70 bar and 200 – 30000 ppm respectively.
RO process
The RO technique has developed greatly in recent decades and has progressed from an emerging technology to become a consolidated, efficient and competitive process. But what exactly is reverse osmosis? To answer this question, we must first describe the osmosis process.
Osmosis is a balancing operation in which molecules in a solvent can cross through a permeable membrane to dilute a more concentrated solution.
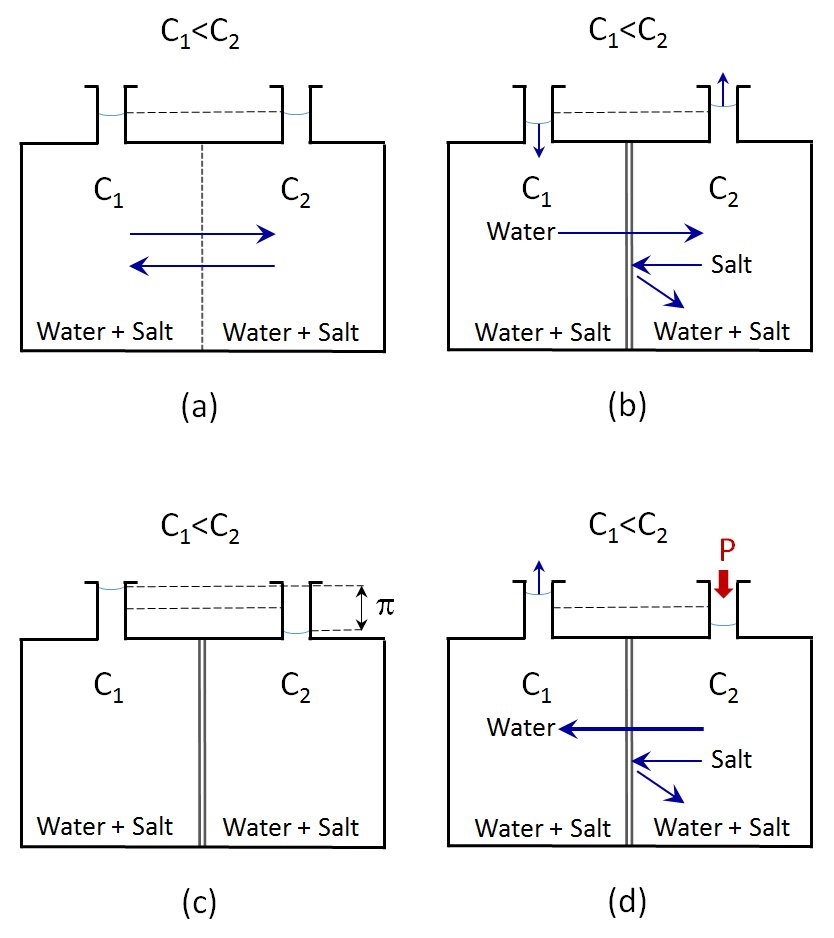
When equipment such as that in Figure (a) is used, in which two solutions with different salt concentrations at atmospheric pressure are separated by a physical barrier, when this barrier separating them is removed there is a natural diffusion and the concentrations in both solutions are equalled out, at which point the balance is reached. Initially, the flow will mostly be from the more dilute solution but as the concentrations equal out, the flows will match and the net flow will be zero.
Figure (b) shows the same experimental system but now the solutions are separated by a semi-permeable membrane through which the solvent can pass but not the larger ions and molecules. In this case the osmosis phenomenon is produced and the more dilute solution passes through the membrane to the more concentrated solution, while the ions in the more concentrated solution cannot pass through the membrane and remain confined.
As a result of this transfer of solvent from one side of the membrane to the other, the levels of both solutions change, as can be seen in the top of the tanks. While the level of the more diluted solution has dropped, that of the more concentrated solution has risen.
Once the flow stops – Figure (c) – and the levels in the two tanks no longer change over time, the system has arrived at balance. The difference in the levels of the liquid in the two tanks generates a hydrostatic pressure exactly equal to the osmotic pressure. In fact, the osmotic pressure is defined as the hydrostatic pressure needed to stop the flow of solvent through a semi-permeable membrane separating two fluids of different concentrations.
When the solvent is flowing from the more dilute solution to the more concentrated solution to equal the concentrations, if a slight pressure is applied to the more concentrated solution, the flow through the membrane reduces.
If the pressure is increased slowly, a point is reached at which the flow through the membrane is zero, that is, the solvent stops flowing through the membrane. The pressure being applied at this moment is equal to the osmotic pressure. If the pressure is increased, the flow reverses and the solvent flows through the membrane in the opposite direction, that is, from the side of the more concentrated solution to the side with the more dilute solution. This process is called RO.
RO thus consists of separating the solvent in a concentrated solution, which passes through a semi-permeable membrane, by applying a pressure that must be at least greater than the osmotic pressure.
The higher the pressure applied, the greater will be the flow permeating through the membrane.
The RO treatment process is especially attractive due to the high selectivity of the membranes, which allow the solvent to pass while the small ions and molecules dissolved in the solution can hardly pass.
This makes the technique especially interesting for a wide variety of applications such as desalinating sea water, treating liquid effluents, purifying water for the food and pharmaceutical industries, etc.
The application of reverse osmosis in the food industry is a clear example where it is widely used; from concentrates of egg whites, fruit juices and gelatins, to removal of bacteria and brine in meat or alcohol removal from spirits. Dairy, starch and sugar industries are also users of the ro plant working process.
Osmosis and RO are two phenomena that occur naturally in living beings in daily life. For example, the cells in our bodies, which are wrapped in a semi-permeable membrane, use osmosis for passing nutrients into and out of the cell, thus favouring both the incorporation of the nutrients needed for the cell’s metabolism and the expulsion of the wastes from the cellular metabolism.
What is the difference between osmosis and reverse osmosis?
In osmosis water molecules move accross a semi-permeable membrane from a higher concentration liquid to a lower one. This requires no extra energy expenditure nor any external process.
In RO, water molucles move across a semi-permeable membrane from a lower concentration liquid to a higher one, which is the reversal of regular osmosis. This requires pressure to be applied in the lower concentration liquid in order to overcome the osmotic pressure, and thus, extra energy.
Types of membranes used in RO
The level of filtration and the process dictates the usage of one type of another of membranes. The most common types of membranes are:
- Spiral membranes
- Ceramic membranes
- Stainless steel membranes
- Tubular membranes
- Hollow fiber membranes
- Plate & frame membranes
Benefits
The benefits of the membranes used in RO can be summarized in the following points:
- The multiple ions retain the single ions better.
- The dissolved gases, such as ammonia, carbon dioxide, sulfur dioxide, oxygen, chlorine and hydrogen sulphide, have a good permeability.
- The rejection of acids and weak bases is greater than pH values when they are in their ionized form.
- The rejection of neutral organic molecules increases with the molecular weight, compounds with molecular weights greater than 100 D have high values of rejection coefficient. The nature of the membrane material has an important influence on the value of this parameter.
Negative values of rejection coefficient have been observed in solutes such as phenol and benzene in cellulose acetate membranes.
Specific contamination problems
Due to the high rejection values in RO processes, contamination is the most important cause of membrane malfunction.
The most frequent causes of contamination are due to:
- Deposits on the surface of the membrane of crusts or scales of calcium carbonate, calcium sulphate, complex silicates, barium sulphate, strontium sulphate, calcium fluoride, etc., depending on the composition of the feed and as a result of the fact that the concentrations of salt in the concentrate can exceed the solubility product of the salt
- Particle sediments such as colloids, products of the corrosion of the iron pipes, precipitates of iron hydroxide, algae, etc.
- Bio-contamination due to the growth of microorganisms on the surface of the membrane, as certain membrane materials such as cellulose acetate or polyamides can be a useful substrate for microorganisms.
- Contamination due to organic compounds such as oil or grease found in industrial wastewater.
The type of cleaning for the membranes will depend on the characteristics of the feed water, the type of membrane and the nature of the contamination. As a general guideline, proceed with alternating periods of rinsing of the membranes, making sure that the cleaning solutions circulate at a high speed over the surface of the membranes with periods in which the membranes are submerged in the cleaning solutions.
The standard cleaning agents used are 1) hydrochloric, phosphoric or citric acids and chelating agents such as EDTA to eliminate saline precipitate crusts, and oxalic acid to eliminate iron sediments 2) alkalis combined with surfactants to eliminate microorganisms, sediments and organic compounds and 3) sterilization of the membranes with chlorine solutions to eliminate microorganisms.
Successive cleaning causes the membranes to deteriorate. Depending on the application, the lifespan guaranteed by the manufacturer is normally 1 – 2 years. With a good cleaning program, the lifespan of the membranes can be extended to 3 years, though a lifespan of 5 years is unlikely.
Applications and uses of RO
The objectives of an RO plant for industrial use are distributed in the following way: 50% in desalination of seawater and brackish water; 40% in the production of ultrapure water for the electronic, pharmaceutical and energy production industries; 10% as decontamination systems for urban and industrial water.
- Desalination of brackish water
The salinity of this type of water is 2000 mg/L – 10000 mg/L. In its treatment, pressures of 14 bar – 21 bar are used to achieve rejection coefficients greater than 90% and to obtain water with saline concentrations of lower than 500 mg/L, which are the values recommended by the WHO as a requirement of potability.
Treatment plants use membrane modules rolled in spirals. It is estimated that the costs for this type of plant are in the region of 0.25 $US/L of treated water/day, with the operating costs being equivalent.
- Desalination of seawater
Depending on the geographical area, the salinity of this type of water is 30000 mg/L – 40000 mg/L. To meet the potability requirements, polyamide hollow fiber membranes are used which allow rejection coefficients to be achieved of greater than 99.3% with working pressures of 50 bar – 70 bar.
The operating costs of this type of treatment plant are estimated to be 1 – 1.25$US/ L of treated water/day, which means that this treatment system is not competitive in comparison with other systems, such as multistage evaporation processes, if water needs exceed 40000 m3 of treated water/day.
- Production of ultrapure water
RO allows water of the quality demanded by the electronic industry to be obtained from drinking water (concentration of dissolved solids < 200 mg/L).
The main problem with this type of installation is the bio-contamination of the membranes, which is why the installation of sterilization systems that use UV radiation is necessary. Table 11 compares the characteristics demanded for drinking water with those for ultrapure water.
- Wastewater treatment
The use of reverse osmosis in wastewater treatment is limited by the high operating costs due to the problems of contamination of the membranes. In the case of industrial wastewater, RO is used in those industries where it is possible to improve the efficiency of the process by recuperating valuable components that can be recycled in the production process: galvanoplasty industries and paint for metal structures, or where reuse of the treated water represents an important reduction in the consumption of water, e.g. textile industry.
In the case of urban water, RO is a treatment that would be indicated as a tertiary treatment, if it were possible for water to be obtained with a quality that made it appropriate for consumption, at a cost of 0.5 – 0.75 $US/m3.
The main problem for the consolidation of this type of treatment is the social reaction. Nevertheless, in areas of Japan and California, where there are extreme water limitations, RO plants are being used to treat water that comes from the biological treatment of domestic water, with the water treated by RO being used to refill groundwater resources.