Sections
- Introduction
- Characteristics of Boron
- Main Global Producers
- Impact of Boron on the Environment
- Effluent Treatment with Boron
- Summary
Introduction
Boron is a metalloid that easily combines in different forms due to having three free electrons in its outer orbital, which gives it a very reactive character. Boron compounds (from the Arabic buraq and this from Persian burah) have been known for thousands of years. In ancient Egypt, mummification depended on natron, a mineral that contained borates and other common salts.
In China, borax crystals were already used around 300 B.C., and in ancient Rome, boron compounds were used in glassmaking. From the 8th century, borates were used in gold and silver refining processes.
In 1808, Humphry Davy, Gay-Lussac, and L. J. Thenard obtained boron with an approximate purity of 50%, although none of them recognized the substance as a new element, which Jöns Jacob Berzelius would do in 1824.
Pure boron was first produced by American chemist W. Weintraub in 1909.
The importance of boron products is reflected in the wide variety of applications they possess. The main uses of borates and boron compounds are: in the enamel and ceramics industry (sanitary ware, tableware, ceramics, tiles), glass industry, crystals and fiberglass, Pyrex glass for utensils, lamps and bulbs, detergents and bleaches, fire retardants, abrasives, cosmetics, wood preservation, capacitors, alloys, catalysis, rubber (flame retardant), cement (slows setting time), fuel (borane for airplanes and rockets), tanneries (prevents putrefaction), pharmacy (mild antiseptic), paints (fungicide), nuclear applications, optics, etc. In addition to these industrial uses, boron is one of the 7 essential micronutrients for plants, making the dosage of boron as a fertilizer extremely important.
Borophene is one of the trendy materials. About 5-6 years ago, graphene seemed to capture global interest at the level of cutting-edge materials for technological development, but currently, borophene is emerging as a strong candidate to take its place, especially in the field of nanotechnology.
Boron in the Earth’s Crust
The estimated concentration of boron in the Earth’s crust is 10 ppm, with a mass of 2.4 × 1017 kg.
It is currently known that boron is much more abundant in sedimentary rocks (300 ppm) than in igneous rocks (3 ppm). This difference is due to four characteristics: boron is sublimable, there is no preference for boron in molten phases (incompatible element), its high mobility in the aqueous phase, and its strong affinity for clay minerals (lithophile element).
Boron reaches the Earth’s crust through different pathways, including atmospheric precipitation, which contains small amounts of boron in solution, and volcanism and analogous geological activity, which release molten rock with variable concentrations of boron.
There are also flows from the ocean to the oceanic crust in the form of sedimentation and diagenesis. The pathways for boron to exit the crust are erosion and plate subduction processes.
Boron tends to concentrate in the residual phases of the molten part; the elements that make up the magma mass solidify based on their melting point and compatibility with the solid phase. Thus, in the successive stages of solidification, the concentration of incompatible elements (including boron) increases in the magma until we finally have a liquid formed by incompatible elements that eventually solidify.
These deposits of incompatible elements are what we know as pegmatites. Following this fact, boron concentrations are relatively low in basalts (6-0.1 ppm) and higher in more crystallized rocks like granite (85 ppm), although high concentrations of boron are also found in granites derived from boron-rich sedimentary rocks. Pegmatites can contain boron concentrations of 1360 ppm.
During the deterioration of submarine rocks, igneous rocks degrade and form clay minerals that adsorb boron from seawater, thus enriching the rock mass in boron.
The basalts of magmatic islands tend to be enriched in boron; this enrichment is attributed to the dehydration of subducted rock blocks rich in boron adsorbed by clay minerals.
Boron-rich fractions participate in the melting process, and the resulting volcanic rocks (andesites and diorites) are consequently enriched in boron. Clay minerals (such as illites, smectites, and montmorillonites) incorporate boron from water both by adsorption and as a substituent element in their structure.
Sedimentary rocks in oceans tend to contain more boron than river sedimentary rocks since seawater has a higher concentration of boron than continental waters.
Boron is only adsorbed at temperatures below 40 °C; at higher temperatures (>150 °C), it can be released from the mineral. Therefore, during the metamorphism of sedimentary rocks, much of the adsorbed boron is released into the water, and if metamorphism increases further, boron as a substituent element is also released. Thus, metamorphic sediments tend to contain significantly lower boron concentrations than their sedimentary rock equivalents.
The main minerals in which we find boron are mostly evaporitic rocks, such as borax, which is highly soluble in water; colemanite; kernite (a partially dehydrated form of borax); and ulexite.
There are also significant boron minerals in the form of igneous rock deposits, such as datolite, tourmaline, and elbaite. These minerals are classified in the borate group (inorganic salts composed of boron and other ions), except for the last two minerals mentioned, which belong to the tourmaline group, appearing especially in pegmatitic veins.
Boron in the Hydrosphere
Boron is found in seawater at estimated concentrations of 4.6 ppm and in a mass of 5.4 × 1015 kg.
It exists as a component of two hydrated molecules: trigonal B(OH)3 and tetrahedral B(OH)4-.
The ratio of the two forms depends on the pH of seawater, and the equilibrium between the concentrations of the two forms is found at a pH of 8.7-8.8. In more basic media, the tetrahedral form predominates, while in more acidic media, the trigonal form prevails.
Due to the long residence time of boron in seawater (25 million years), the concentrations of B(OH)3 and B(OH)4- do not vary significantly across different oceans.
Boron reaches the hydrosphere from the continents through the water cycle and by processes of rock erosion, and from the oceanic crust through hydrothermal circulation, as well as from atmospheric precipitation.
Boron in the Atmosphere
The atmosphere contains about 2.7 × 108 kg of boron. It is found in the troposphere in a gaseous state 97% of the time; the remaining 3% is in a solid state as particles.
The residence times considered for tropospheric boron in its gaseous form are 19 to 36 days, while for particulate boron, they are 2 to 6 days. Due to these low residence times, boron concentrations are variable at different points in the atmosphere.
Boron reaches the atmosphere through the evaporation of seawater and can return to the oceans or continents through precipitation.
Boron in Plants
For plants, boron is an essential nutrient. It seems to play a fundamental role in maintaining the structure of the cell wall (through the formation of cis-diol groups) and membranes.
It is a poorly mobile element in the phloem, which is why deficiency symptoms usually appear in young leaves, while toxicity symptoms appear in mature leaves.
An excess of boron is harmful to some plants that are less tolerant to this element, potentially weakening their veins. In apple and pear trees, boron deficiency manifests in the fruits as internal malformation.
Characteristics of Boron
The main physical and chemical characteristics of boron are as follows:
| Name, symbol, number | Boron, B, 5 |
| Chemical series | Metalloids |
| Group, period, block | 13, 2, p |
| Atomic mass | 10.811(7) u |
| Electronic configuration | [He]2s22p1 |
| Mohs hardness | 9.5 |
| Electrons per level | 2, 3 |
| Mean radius | 85 pm |
| Electronegativity | 2.04 (Pauling scale) |
| Calculated atomic radius | 87 pm (Bohr radius) |
| Covalent radius | 82 pm |
| Oxidation state(s) | 3 (slightly acidic) |
| Ordinary state | Solid (non-magnetic) |
| Density | 2460 kg/m3 |
| Melting point | 2349 K (2076 °C) |
| Boiling point | 4200 K (3927 °C) |
Boron exhibits a multitude of allotropes that have a regular icosahedron as a common structural element. The arrangement of the icosahedra can be in two distinct forms:
- Joining two icosahedra by two vertices, through normal covalent B – B bonds
- Joining three icosahedra by three vertices, through a three-center bond with two electrons.
Within these possible unions, in crystalline boron, the icosahedra can associate in various ways to create the corresponding allotropes:
- Tetragonal Boron (T – 50): formed by 50 boron atoms per unit cell, which are four icosahedral units joined together by some B – B bonds and two elemental borons that act as tetrahedral connections between icosahedra. It has a density of 2.31 g/cm3.
- Alpha Rhombohedral Boron (R – 12): formed by sheets of icosahedra joined in parallel. The intralaminar unions are made through three-center bonds, while the interlaminar unions occur through two-center bonds. The density of this type of boron is 2.46 g/cm3, and it has a light red color.
- Beta Rhombohedral Boron (R – 105): formed by twelve B12 icosahedra arranged in an icosahedral shape around a central B12 unit, that is, B12(B12)12. It has a density of 2.35 g/cm3.
Isotopes of Boron
In nature, there are two isotopes of boron, 11B (80.1%) and 10B (19.9%).
The results of their masses differ across a wide range of values defined as the difference between the fractions of 11B and 10B, traditionally expressed in parts per thousand, in natural waters ranging from -16 to 59.
There are 13 known isotopes of boron, with the shortest-lived isotope being 7B, which decays through proton emission and alpha decay.
It has a half-life of 3.5×10−22s. The isotopic fractionation of boron is controlled by the exchange reactions of the special compounds B(OH)3 and B(OH)4.
Boron isotopes are also fractionated during the crystallization of minerals, during phase changes of H2O in hydrothermal systems, and during the hydrothermal alteration of rocks.
Main Global Producers
Boron originates from various compounds, from simple oxides to very complex polymeric structures.
Among them are the oxides known as borates. Commercially significant borate deposits are located only in a limited number of geographical regions worldwide: Anatolia (Turkey), California and Nevada (southwest United States), the South American Puna (southern Peru, southwestern Bolivia, northern Chile, and northwestern Argentina), Inder (Russia), and Central Asia (China and Russia).
The South American Puna has the third-largest borate reserves in the world, after Turkey and the west coast of the United States.
The deposits in the South American Puna preferentially produce ulexite, tincal, colemanite, and hydroboracite. These constitute the useful mineral of commercial value, which is found mixed with other worthless materials that make up the gangue, from which it must be separated.
Ulexite is a sodium and calcium borate, poorly soluble in cold water, accompanied by gangue consisting of sands, clays, gypsum, calcite, all impregnated in a brine composed mainly of sodium chlorides and sulfates. The borates known as “hard” (tincal, colemanite, and hydroboracite) have gangue made up of rocks (calcite, dragonite, clay, tuffs, tufas) and iron impurities.
Tincal is a sodium borate, soluble in water, a property that is used to separate it from the insoluble gangue and subsequently obtain borax through cooling crystallization. Colemanite is a calcium borate with five water molecules in its structure, and hydroboracite is a calcium and magnesium borate with six water molecules. Both have iron and arsenic as their main impurities.
Impact of Boron on the Environment
Neither boron nor borates are toxic to humans and animals. The LD50 for animals is about 6 g per kg of body weight. Substances with an LD50 above 2g are considered non-toxic.
The minimum lethal dose for humans has not been established, but a consumption of 4 g/day has been reported without incidents, and clinical doses of 20 g of boric acid for neutron capture therapy did not cause problems.
Some fish have survived for 30 minutes in a saturated boric acid solution and can survive longer in borax solutions. Borates are more toxic to insects than to mammals.
Boranes and some similar gaseous compounds are highly toxic. It is not an element that is intrinsically poisonous, but its toxicity depends on the structure.
Boranes (hydrogen boron compounds) are toxic, as well as easily flammable, and require special care during handling. Sodium borohydride presents a fire hazard due to its reducing nature and the release of hydrogen upon contact with acid. Boron halides are corrosive.
Boron in Human Health
Scientifically, it has not been demonstrated that boron is a substance considered essential in the human diet or that it is a dietary requirement in vertebrates and invertebrates, or at least of the same importance as it occupies in plants.
The human body contains at least 0.7 mg of boron per kilogram of weight obtained from the consumption of water and vegetables. A human consumes about 0.8 to 2.5 mg of boron per kilogram of weight in their daily intake without any symptoms manifesting.
Forced diets of 5 g per day can cause nausea, diarrhea, and vomiting; some authors suggest that 20 g per day of boron may be lethal in sensitive organisms, but this has not been proven.
Other literature seems to associate the onset of arthritis with the intake of this element, and other publications estimate that this element should be considered at the level of an essential element for the metabolism of calcium, copper, magnesium, and nitrogen fixation.
Boron can be toxic to plants, even at low concentration levels. A boron concentration below 1 mg/l is essential for plant development. Most plants show toxicity problems when the boron concentration exceeds 2 mg/l.
The World Health Organization recommends a boron concentration in drinking water of less than 0.5 mg/l. EU standards require a boron level of less than 1 mg/l.
Effluent Treatment with Boron
Boron, due to its nature, is not easy to remove from aqueous matrices. Classical techniques of coagulation, sedimentation, and even reverse osmosis are not satisfactory.
Some experiences have indicated that the application of ion exchange resin systems in conjunction with zeolites and activated carbon is much more promising as a way to reduce this element.
Table of Common Treatments for Effluents with Boron
| Method | Initial Boron Concentration | Effectiveness | Process | Industrial Application | Relative Costs |
| Alkaline Precipitation | High | Low | Discontinuous | Low | Very low |
| Adsorption Me(OH)x | High | Very high | Discontinuous | High | Low |
| Adsorption on Clay | High | Very high | Discontinuous | Medium | Low |
| Ion Exchange | Low | Very high | Continuous (regeneration) | High | High |
| Extraction | Low | High | Continuous | Low | High |
| Reverse Osmosis | Low | High | Continuous (cleaning) | High | High |
| Electrodialysis | Low | High | Continuous | Low | High |
| Evaporation | High | High | Continuous | Medium | High |
The most commonly used solutions are: Reverse Osmosis, Ion Exchange, and Evaporation treatment of effluents.
Desalination by Reverse Osmosis
The amount of boron in seawater varies from 4 to 5.5 mg/L, proportional to salinity. It mainly comes from the discharge of wastewater treatment plants, where soaps and detergents begin to be used, as well as from agricultural fertilizers.
Boron is present in water as boric acid H3BO3 and borate H3BO2-. The predominant boron species depends on the pH of the water.
The pKa value of H3BO3/H3BO2- is 9.2, therefore, the equilibrium is normally shifted to the left, as the standard pH value of seawater is 8.
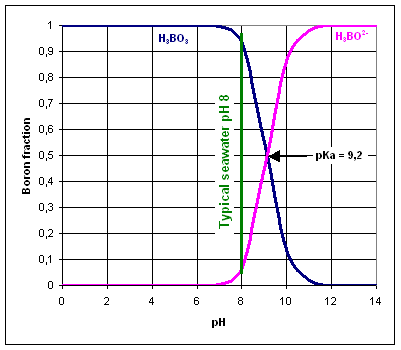
Reverse osmosis membranes are very efficient in removing charged species like the borate ion, rather than neutral molecules like boric acid.
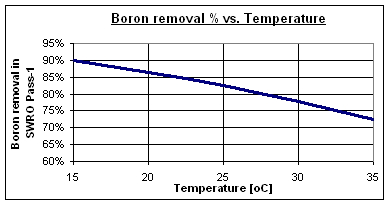
Typical boron removal rates at pH 8 are between 73 and 90% for standard high-rejection seawater reverse osmosis membranes, depending on the water temperature. Some special high-boron-removal membranes can achieve values of up to 95%.
Normally, high salinity seawater has a high boron content and is found in areas with very warm climates, such as the Persian Gulf, the Red Sea, the eastern Mediterranean Sea, and the Caribbean Sea.
At 30 °C, boron removal is reduced to 78%, leaving 1.15 mg/l in the permeate stream of Step 1. Therefore, a specific boron removal process is necessary to reach the 0.5 mg/l required by the WHO.
Removal of Boron from Desalinated Water
There are two main processes to produce drinking water with less than 0.5 mg/L of boron, depending on the salinity of the water, the concentration of boron, and the temperature.
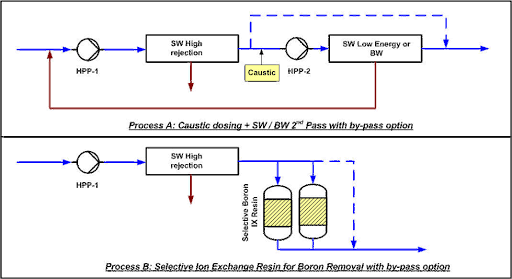
Process A: SWRO in 2 Steps:
In Step 2 of reverse osmosis, caustic soda is added to raise the pH to 9.5. Part of the permeate from Step 1 may be bypassed to maintain a certain amount of minerals in the water. The second step may consist of low-energy seawater membranes if the temperature and salinity are high or high-rejection brackish water membranes in less severe conditions.
Process B: SWRO + IX:
An ion exchange resin is added with or without bypass, depending on the concentration of boron and the specific requirements of the treatment process.Here is the translated text with the HTML tags preserved:
The necessary residual boron removal. The resin, which must be selective for chlorine, is regenerated in-situ with caustic soda and hydrochloric acid. For continuous production, a dual-column system is required.
The boron removal treatment system using selective ion exchange allows for the elimination of excess boron from any water, which, in certain crops (mainly stone or pome fruit), can reach toxic or harmful concentrations for the plantation.
This toxicity can be more pronounced when using wastewater for irrigation or groundwater contaminated by the former.
The influent passes through the specially designed ion exchange resin for the removal of boron in aqueous solutions, resulting in water with a 90% reduction in boron at the outlet.
The exchange capacity is limited, and when the resin has reached its exhaustion, regeneration is performed.
The regeneration of the resins is fully automated and is carried out by passing a specific concentration of acid through the resin bed, removing the retained boron, allowing for the storage of the regeneration aqueous solution, so that it can be managed as waste later.
Boron-rich effluents from resins or reverse osmosis membranes can be concentrated to values that allow for their recovery, using vacuum evaporation techniques. Condorchem – Envitech has the capacity to offer comprehensive solutions for this application.
Summary
Boron is a highly reactive chemical element that appears in nature combined in various forms. It has multiple industrial applications and an important future based on the emergence of borophene, as a successor to graphene, whose properties make it a strong candidate for nanotechnology applications.
Although it is necessary for plant life and is well tolerated by humans and animals, there are limits that should not be exceeded to avoid becoming harmful.
One of the technical solutions that has been used for many years to combat drought is desalination by reverse osmosis, but the concentrate is rich in this element, and the desalinated water often remains above the 0.5 ppm limit established by the WHO, which requires additional treatments to separate more boron.
Thus, the options consist of passing the permeate through a second reverse osmosis step or through a bed of specific resins. In the case of resins, the vast majority of the present boron is removed.
In both cases, a concentrate remains that can be recovered after concentration in a vacuum evaporation system, in which Condorchem – Envitec has proven experience.
Bibliographic References and Internet Links
http://oa.upm.es/155/1/05200006.pdf
https://rua.ua.es/dspace/bitstream/10045/13838/1/Tesis_Chillon.pdfçç