Sections
- Introduction
- The cosmetic industry
- The chemistry of cosmetics
- Treatment of effluents from the cosmetic industry
- Summary
Introduction
The word “cosmetics” derives from the Greek “kosmetikós” (related to ornamentation) and encompasses all those products that humans have been using since prehistoric times for their appearance and personal hygiene.
Cosmetic products were initially handmade, but due to their growing demand and evolution, they have become one of the most flourishing industrial sectors in the market.
As we will see in later chapters, the production of these products involves the use of a multitude of organic and inorganic compounds, many of which are toxic, resulting in strong contamination of the effluents generated in the factories that produce them.
Many of these compounds can be separated from the wastewater by conventional processes such as physicochemical treatments (sedimentation/flotation/filtration, etc.) and aerobic or anaerobic biological treatments. However, others contain high concentrations of refractory COD, so more complex treatments must be applied to meet legal requirements for wastewater discharge.
This article mentions the most common compounds used in the production of cosmetics, as well as the different treatment options currently used for the purification of their wastewater.
It also indicates the applicability of the most advanced technologies, such as POA’s, and the consideration of the vacuum evaporation process, applicable both for the recovery and treatment of segregated effluents and for the treatment of overall wastewater discharge.
Condorchem Envitech is a company specialized in the design and construction of wastewater treatment plants that offers studied and tailored solutions for each case.
The cosmetic industry
Giant cosmetic companies generate billions of dollars annually and were founded in the 20th century by chemists and pharmacists in the United States and France.
Currently, non-toxic and hypoallergenic products are sought to replace those that were used for many years as skin lighteners and firming agents, which were composed of dangerous chemical elements such as mercury, lead, and arsenic, until they were banned due to their toxicity.
The current cosmetic industry involves branches such as chemistry, biology, pharmacy, and medicine.
The definition provided by the Food and Drug Administration (FDA) states that a cosmetic is a “substance intended to be applied to the human body to cleanse, beautify, or alter appearance without affecting the body’s structure or functions.”
Cosmetic products are made following a formula that involves four components, which are:
- Active ingredient
- Excipient or vehicle
- Additives
- Correctors
Following this base, products for various applications are created, including creams or lotions for skincare, treatments to conceal skin imperfections, lip and nail color products, etc.
In general terms, the subsectors covered by the cosmetic industry are:
- Perfumes and fragrances
- Decorative or color cosmetics (makeup)
- Skincare products
- Haircare products
- Cleaning and hygiene products
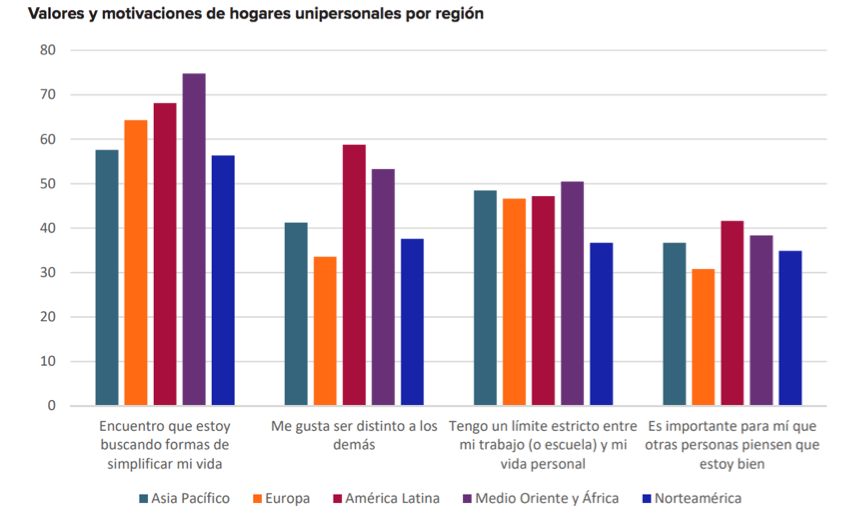
According to each world region and its cultural influences, there are psychological trends that encourage the consumption of different products, depending on their priority order, as indicated in the following graph, which is based on a statistical study conducted by the main companies in the sector:
The chemistry of cosmetics
The basic ingredients most commonly used in cosmetics serve a specific function in the composition of the product.
These include preservatives that extend their shelf life, emulsifiers that help integrate liquids with fats, bactericides that prevent the formation of microorganisms such as fungi, antioxidants that prevent the mixture from oxidizing in contact with air, and gelling agents that give it a creamy texture.
The chemical compounds used to achieve the mentioned effects of the ingredients can vary considerably depending on the origin of the product and its cost. Animal or plant-based compounds are also used. Some of the compounds used are:
- Phenol and phenyl
- Siloxanes
- Phthalates
- Butylated hydroxyanisole (BHA)
- Diethanolamine (DEA)
- Formaldehyde
- Mineral oils
- Other ingredients derived from petroleum include methysilanol, ozokerite, microcrystalline wax, vaseline, ceresin, and propylene glycol
- Triclosan
- Sodium lauryl sulfate
- Colorants
- Aluminum
- Lactic acid
- Casein
- Adrenaline
- Beeswax
- Propolis
- Albumin
- Biotin
- Silk
- Spermaceti
- Collagen
- Keratin
- Glycerol or glycerin
- Marine oil
- Gelatin
- Turtle oil
- Musk oil
- Lactose
- Uric acid
- Provitamin A, beta-carotene, b-carotene, or carotene
- Panthenol
Treatment of effluents from the cosmetic industry
Given the high diversity of products that can be manufactured in the cosmetics industry and their varied characteristics and potential toxicities, it is very difficult to characterize a specific wastewater treatment; however, after reviewing various effluent analyses, the following has been taken as representative of an industry of this type:
Typical analytical analysis for industrial cosmetic wastewater
| Parameter | Concentration in mg/l (before TSS) | Concentration in mg/l (after TSS) |
| BOD5 | 1910 | 1495 |
| COD | 3436 | 2720 |
| Suspended Solids (SS) | 980 | 124 |
| Biodegradability | – | 0.39 |
| pH | 7 | 8 |
| Surfactants | >100 | 47.5 |
| Oils and fats | >100 | 32 |
A common treatment, which is usually implemented as a primary treatment, is physicochemical treatment, aimed at removing settleable and floatable materials, followed by a secondary treatment that is usually specific to each type of effluent.
Biological treatment, whether aerobic or anaerobic, only yields good results for more biodegradable effluents. In the case of less biodegradable effluents, it is common to use chemical oxidation treatment systems, such as POA’s or vacuum evaporation.
Vacuum evaporation is particularly applicable when the factory has excess energy, either in the form of steam or hot water, and the effluents can be segregated and treated in such a way that the concentrations of the final discharge are significantly reduced.
Summary
The global production of the cosmetic industry has been steadily increasing due to the growing demand for products to delay or correct the physical signs of natural aging.
However, some of the components used to manufacture these products are often toxic, making it difficult to treat the resulting waste and effluents using conventional methods.
The typical treatment line consists of a physicochemical treatment at the beginning, aimed at removing settleable and floatable materials, followed by a secondary treatment that is usually specific to each type of effluent.
Biological processes, whether aerobic or anaerobic, only yield good results for more biodegradable effluents. In the case of less biodegradable effluents, it is common to use chemical oxidation treatment systems, such as POA’s, or vacuum evaporation.
The goal of effluent treatment is to adjust its composition to comply with current legislation. There are various types of treatment, and it is often necessary to design an integrated system of units to ensure the required level of purification.
Wastewater treatment can be carried out using different techniques, taking into account the specific characteristics of the wastewater. These techniques can be grouped into two general types of solutions.
On one hand, there are the so-called treatments without conversion, which simply involve the transfer of contaminants, and those in which these contaminants are transformed into harmless species.
Treatments without conversion are physical separation methods in which the contaminant is concentrated or isolated for subsequent recovery or easier disposal. Within this group, adsorption on activated carbon stands out, based on the transfer of contaminants from the liquid phase to the surface. The advantages of using activated carbon over other adsorbents lie in its high adsorption capacity and chemical stability.
The main disadvantage of adsorption is the transfer of the pollution problem to the adsorbent, which becomes a waste that requires proper management. When the adsorbent is regenerated, the retained contaminants pass into another liquid or gaseous phase, which needs to be treated.
Desorption, carried out by air stripping, is another non-conversion technique useful for removing volatile contaminants from water, generating a residual gas stream that must be properly treated before being released into the atmosphere.
In this sense, the combination of air stripping and gas-phase adsorption is a solution that, in many cases, is better than direct liquid-phase adsorption. Another non-conversion technique is liquid-liquid extraction.
It is an economical and relatively effective method of recovery when the concentration of contaminants is high. However, solvent losses can be significant, creating an additional pollution problem.
Treatments with conversion: The limitations of non-conversion treatments require the development of more effective procedures for the treatment of industrial wastewater.
Conversion techniques can be grouped into three main categories: thermal treatments, biological treatments, and chemical treatments, which include oxidation and reduction processes.
Thermal methods: Among thermal treatments, we mention incineration, which is used for small volumes of wastewater with a high organic load, exceeding 100 g/L of COD. The economy of the process is determined by the additional fuel consumption required to maintain the process. Along with the high cost, the main disadvantage is the presence of highly toxic oxidation products (dioxins, furans, etc.) in the combustion gases, which are much more toxic than the original contaminants.
Vacuum evaporation, applicable for relatively small flows (<20 m3/h), is a more cost-effective treatment, especially if there is excess heat available, and the gases mostly condense with the water vapor. It is highly recommended in factories that frequently change their production, resulting in significant production tails that can be recovered depending on the product.
The volatiles that migrate to the condensate are usually organic and can be treated by salt separation processes (reverse osmosis, nanofiltration, etc.) or by adsorption with activated carbon. Once treated, this condensate can be reused as service water and/or process water.
While it is true that biological methods are highly effective for the removal of a wide range of contaminants, they have some disadvantages. Biodegradation is a slow process that does not allow for a high degree of contaminant removal if the concentration is high, and it is not suitable for treating industrial effluents containing compounds toxic to microorganisms, as is often the case in the cosmetic industry.
Oxidation treatments with air or O2 and activated sludge may not be efficient due to the low biodegradability of the effluents. On the other hand, anaerobic reactors such as UASB or upflow anaerobic sludge blanket reactors have been used for the treatment of complex effluents generated by this type of industry, but the results have not always been satisfactory.
Chemical methods include both reduction and oxidation processes. Chemical reduction has been widely applied in industrial processes, involving the use of a reducing agent, usually at high pressure and temperature and with an appropriate catalyst.
In most cases, hydrogen is used as the reducing agent, but there are others, such as metal hydrides, formic acid and its salts, hydrazine, and alkoxides. The most commonly used reduction treatments in industry are hydrogenation, hydrodesulfurization, hydrodenitrification, hydrodeoxygenation, and hydrodehalogenation.
In general, these processes (except for hydrodechlorination and to a lesser extent hydrodenitrification) have not been applied to the removal of contaminants in water. Hydrodechlorination involves breaking the carbon-chlorine bond of a chlorinated organic molecule through hydrogenation, converting it into the corresponding organic compound without chlorine, which is then eliminated as HCl.
The use of a catalyst is essential, with noble metals (palladium, platinum, and rhodium) supported on activated carbon, alumina, or zeolites being the most common catalysts. Hydrodechlorination using Pd catalysts supported on activated carbon has been used for the removal of various compounds in wastewater, such as chlorinated hydrocarbons (carbon tetrachloride, chloroform, trichloroethylene, trichlorobenzene, etc.) and chlorinated phenolic compounds.
In industrialized countries, the use of Advanced Oxidation Processes (AOPs) is increasingly widespread. The concept was initially established by Glaze et al. (1987), who defined AOPs as processes involving the generation and use of transient species with high oxidative potential, mainly the hydroxyl radical (HO–), under practically ambient conditions.
These radicals can be generated by photochemical means (including sunlight) or by other forms of energy. They have a high oxidative power and react with organic matter at much higher rates than alternative oxidants such as ozone.
Another fundamental characteristic of hydroxyl radicals is their low selectivity, which is a very important property for their use in wastewater treatment. AOPs can be used alone or in combination with each other or with conventional methods, and can also be applied to contaminants in air and soil.
They also provide disinfection of treated water by inactivating bacteria and viruses. There are many different contaminants that can be degraded using these techniques.
However, simpler compounds such as oxalic acid, acetic acid, or halogenated derivatives like chloroform or tetrachloroethylene are resistant to this treatment. Some typical compounds that can be oxidized by the OH– group include organic acids, alcohols, aldehydes, aromatics, amines, diazo compounds, ethers, ketones, etc.
Diagram of the cosmetic industry effluent treatment line
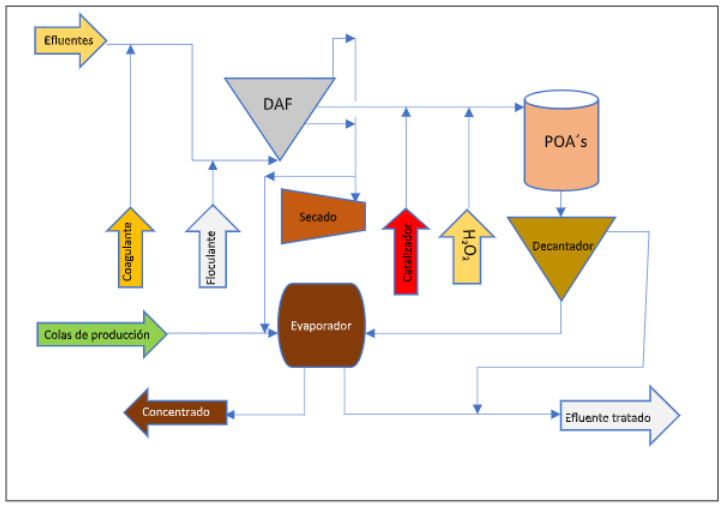
Effluent treatment by advanced oxidation (AOP)
Summary
The global production of the cosmetic industry has been steadily increasing due to the growing demand for products to delay or correct the physical signs of natural aging.
However, some of the components used to manufacture these products are often toxic, making it difficult to treat the resulting waste and effluents using conventional methods.
The typical treatment line consists of a physicochemical treatment at the beginning, aimed at removing settleable and floatable materials, followed by a secondary treatment that is usually specific to each type of effluent.
The biological process, whether aerobic or anaerobic, only yields good results for more biodegradable effluents. In the case of less biodegradable effluents, it is common to use chemical oxidation treatment systems, such as AOPs, or vacuum evaporation.
Vacuum evaporation is particularly applicable when the factory has excess energy, either in the form of steam or hot water, and the effluents can be segregated and treated in such a way that the concentrations of the final discharge are significantly reduced.
Bibliography and Internet references
https://www.uco.es/idep/images/documentos/masteres/comercio-exterior-internacionalizacion/ejemplo-tfm-comercio.pdf
https://www.elmundo.es/economia/2017/07/24/5968bab046163f54588b4631.html
https://forbes.es/empresas/43235/la-cosmetica-ya-no-solo-tiene-que-ver-con-la-estetica/
https://iquimicas.com/composicion-quimica-de-los-cosmeticos/