Sections
- Lithium extraction and refining systems
- Introduction to lithium
- Lithium applications
- Lithium extraction processes
- Precipitation and refining of lithium chloride
- Vacuum evaporators and crystallizers for lithium refining
Evaporators and crystallizers for lithium refining
Condorchem Enviro Solutions offers a set of high-efficiency crystallization solutions for the purification of lithium. We deliver efficient, durable and reliable evaporators and crystallizers to optimize lithium refining processes.
Our evapo-crystallization solutions for the concentration and refining of lithium are divided into the following processes:
- Design of a customized lithium salt extraction process.
- Extraction and physicochemical purification of lithium effluents.
- Concentration and crystallization of lithium salts.
- Laboratory tests: Matter balance and efficiencies.
- Demonstration industrial pilot plants.
Additionally we supply comprehensive solutions for the recovery of lithium, as well as other metals, in the recycling of lithium ion batteries.
Introduction to lithium
Lithium occurs naturally in approximately 145 different minerals, although only a few contain concentrations that can be considered commercially viable. It is also present in brines, hot springs, and seawater, in widely varying amounts ranging from 20 ppm to 65 ppm.
This element can be found in a variety of forms, including: anomalous concentrations in pegmatites; sedimentary environments associated with clays; hydrothermal alteration zones associated with minerals formed at both low and high temperatures; non-marine evaporites; brines from desert environments; saline waters or brines associated with petroleum deposits; boron, beryllium, fluorine, manganese, and possibly phosphate deposits; lacustrine environments associated with magnesium silicates; waters, plants, and soils in desert climates; and iron-rich sedimentary rocks.

It should be noted that the main deposits currently in production are located in pegmatites or in brines from paleo-lacustrine saline deposits. In most cases, exploration indicators merely confirm the presence of anomalous lithium concentrations, without sufficient grades for economic recovery under current market conditions.
Lithium has multiple applications, including: the manufacture of batteries for computers, mobile phones, and electric vehicles (demand for the latter is expected to increase sharply with mass production); pharmaceutical products for the treatment of neurological disorders (antidepressants); ambient air purification; magnesium–lithium alloys for aerospace applications; lithium-based industrial lubricants; use in the nuclear industry as a coolant pH regulator; and tritium production for future generations of nuclear fusion reactors.
Lithium carbonate (Li₂CO₃) is the most widely used lithium compound; one gram of lithium is contained in 5.32 grams of lithium carbonate.
Each year, in Japan alone, around 10,000 new materials are studied and documented, with distinct physical, chemical, electrical, magnetic, ionic, and electrochemical properties. Future developments are expected to include new products such as lithium cyanide, lithium hydroxide, and metallic lithium.
Lithium applications
Lithium’s first commercial markets were metallurgy (light Al–Li and Pb alloys), ceramics & glass (Li₂CO₃ as a flux), air‑conditioning (LiBr absorption chillers), and organolithium reagents for polymers and fine chemicals. These legacy segments still exist, but they are now small compared with batteries, which drive the vast majority of demand.
Today’s demand drivers (2025):
- Batteries account for roughly 85–87% of global lithium use
- Ceramics & glass ~4–5%
- Lubricating greases ~2%
- The remainder spans air treatment, continuous-casting fluxes, medical, and other niche uses.
This “batteries dominance” has huge implications for companies providing lithium refining solutions as battery supply chains typically buy Li₂CO₃ or LiOH·H₂O as qualified products. Chemistry choice depends on cathode technology:
- LFP value chains generally specify lithium carbonate.
- Nickel-rich cathodes (NMC/NCA) generally specify lithium hydroxide.
Crystallization systems (primary treatment + vacuum recrystallization + washing) are critical to meet battery‑grade purity (≥99.5–99.9%), particle‑size distribution (PSD), and ultra‑low Mg, Ca, Na/K, B, regardless of the lithium source (ponds, DLE eluate, or hard‑rock leachate).

- Batteries (EVs + stationary storage) are the growth engine of lithium market. Rising LFP adoption in EVs and stationary storage continues to pull significant volumes of Li₂CO₃, while high‑nickel platforms keep LiOH strategically important. For refiners this implies:
- Maintaining capability on both carbonate and hydroxide lines.
- Using vacuum recrystallization and counter‑current cold washes to push contaminants below spec.
- Designing for mother‑liquor recycle and by‑product crystallization (e.g., Na₂SO₄).
- Improve yield and ESG metrics.
- Ceramics & glass are still a meaningful non‑battery outlet, with a modest share of total demand. Many applications accept technical‑grade Li₂CO₃, but premium segments (e.g., specialty glass) benefit from tighter impurity control and consistent PSD, which can only be achieved with controlled crystallization.
- Lithium stearate greases (lubricating greases) remain widely used for high‑temperature applications. While purity thresholds are generally lower than for batteries, stable supply and cost predictability matter. In this context, crystallizer integration with water reuse supports both OPEX and ESG positioning for these lower‑spec products.
- A small portion of the demand goes to specification-driven applications such as:
- Air treatment & specialty chemicals.
- LiBr solutions (absorption chillers).
- Dehumidification.
- Continuous‑casting mold fluxes.
- Medical/specialty uses
- Nuclear: Primary‑circuit chemistry in light‑water reactors typically uses boric acid + LiOH; some VVER plants have historically used KOH, and KOH has been studied for PWRs. This is niche relative to batteries and does not materially affect lithium salt demand today.
To sum up:
- Batteries dominate demand; Li₂CO₃ vs LiOH depends on cathode chemistry
- Vacuum crystallization (plus impurity polishing and mother‑liquor management) is what consistently converts diverse feeds into battery‑grade product with attractive ESG metrics.
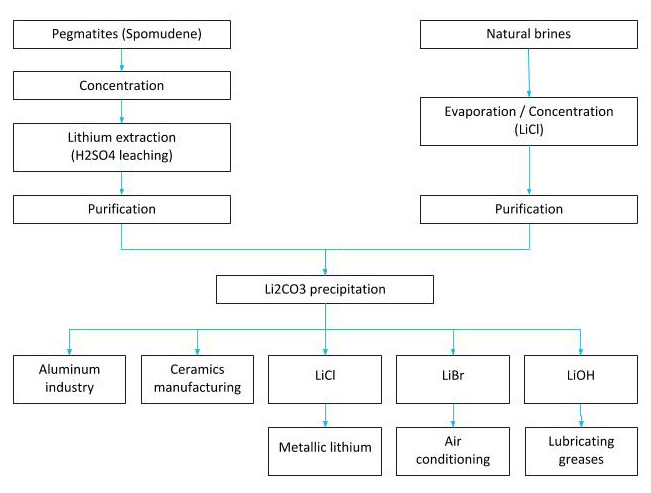
Lithium extraction processes
Lithium is extracted from brines containing natural salts, such as those in the Salar de Atacama in Chile, the Salar del Hombre Muerto and other sites in Argentina, as well as from mineral deposits such as the Greenbush spodumene deposit in Australia, and from one of the largest known lithium reserves in the world (21 million metric tons) in Bolivia’s Salar de Uyuni.
A large share of global lithium production comes from brines, whose production cost is much lower than that of mineral deposits (according to John McNulty, USD 1,500–2,300 per ton from brines versus USD 4,200–4,500 per ton from mineral ores).
Lithium is obtained from two primary natural sources.
- The first is the mineral spodumene, a lithium aluminum double silicate (LiAlSi₂O₆) typically found in association with quartz, mica, and feldspar.
- The second source is natural brines from salt flats and geysers, where lithium occurs primarily as lithium salts, mainly lithium potassium double sulfate (KLiSO₄).
As noted above, lithium can be obtained from deposits of lithium aluminum double silicate (LiAlSi₂O₆ or Li₂O·Al₂O₃·4SiO₂), which contain 3.73% Li and, as oxide, 8.03% Li₂O. The other constituent elements are present in the following proportions: 51.59% oxygen; 30.18% silicon (as silicon oxide, 64.58% SiO₂); and 14.5% aluminum (as aluminum oxide, 27.4% Al₂O₃).
Spodumene is a crystal with a Mohs hardness of 6.5 to 7 and a density of 3.1 g/cm³. It is notable for its range of colors, which can vary from light gray, yellow, and green to purple.
Bolivia’s lithium reserves or resources are contained in brines with an approximate density of 1.2 grams per liter (g/L). Thus, a lithium concentration of 0.1% by weight is equivalent to 1,000 parts per million (ppm) and corresponds to 1.2 g/L of lithium salt concentration.
Lithium brine extraction is carried out by pumping, and lithium concentration can be increased using two main processes.
1. The first is adsorption using a selective lithium adsorbent (polyethylene glycol).
Adsorption offers advantages such as:
- Being unaffected by the composition of the saline water (it can process brines with low lithium concentrations, as has been demonstrated experimentally with seawater).
- Independence from local weather conditions.
- Minimal waste generation.
However, it has drawbacks: the need for chemical reagents, expensive and complex adsorption equipment, and the high cost of the adsorbent.
2. The second is evaporation in shallow ponds constructed for this purpose. Evaporation not only increases the concentration of salts but also causes some of them to precipitate once saturation is reached.
The advantages of natural evaporation include:
- Zero energy consumption
- Low use of chemical reagents.
Its disadvantages are the need to use an additional separation method, the accumulation of waste, and dependence on local weather conditions, specifically evaporation rate and rainfall.
3. Direct Lithium Extraction (DLE) is a process that pulls lithium from brine without extensive evaporation. Techniques under this umbrella include ion-exchange sorbents, solvent extraction, selective membranes, and electrochemical lithium pumping
DLE can vastly speed up lithium recovery and reduce land use. However, DLE usually yields a concentrated lithium solution, such as lithium chloride in water (LiCl/Li₂SO₄), rather than a final solid product. That’s why crystallization is needed to convert that lithium-rich solution into lithium carbonate or hydroxide after DLE.
DLE can replace evaporation ponds, but not the refining steps. Vacuum crystallizers turn DLE outputs into saleable battery-grade salts.
Moreover, many DLE methods work best at extracting lithium but may co-produce impurities or require chemical tweaks (pH adjustments, etc.), so a final crystallization/purification step is crucial. It’s also noteworthy that some DLE implementations still involve thermal steps (e.g. thermal-assisted adsorption or membrane distillation) which are essentially controlled evaporation
Advantatges of DLE:
- DLE avoids large-scale evaporation, as it utilizes technologies like ion exchange, solvent extraction, or membrane filtration to directly extract lithium from the brine without the need for extensive evaporation.
- DLE can extract lithium in a matter of hours or days, compared to the months or years required for traditional evaporation methods.
- DLE can achieve higher lithium recovery rates (potentially 70-90%) compared to evaporation (40-60%).
- DLE often requires less land, less water, and less energy than traditional methods, potentially leading to a smaller environmental impact.
- DLE technologies are designed to selectively target and extract lithium ions, minimizing the extraction of other elements from the brine.
In essence, DLE represents a technological shift in lithium extraction, offering a more efficient, faster, and potentially more sustainable approach compared to traditional methods that rely heavily on evaporation.
4. Solvent Extraction and Ion Exchange is a method to selectively separate lithium or impurities using chemical reagents. For example, solvent extraction can remove contaminants like boron from brine before lithium precipitation
Ion-exchange resins can polish lithium solutions by capturing calcium or magnesium. While effective for purification, these methods alone do not yield a final lithium salt. They produce a cleaned-up lithium solution that still needs concentration and crystallization to produce the solid lithium compound.
In practice, solvent extraction and ion exchange are often combined with crystallization in a flowsheet. Thus, crystallizers work in tandem with chemical extractants: the former delivering the physical separation (solid product formation) after the latter achieve chemical separation (selectivity).
Evaporation vs adsorption comparison
The latter method has been chosen for the Salar de Uyuni, where the installed pilot plant will operate.
Most of the world’s lithium production comes from the brines of the Salar de Atacama in Chile, where evaporation is used. Data and numerous operating parameters from this site allow for comparison with conditions in the Salar de Uyuni.
- Atacama brines are richer in lithium (as well as potassium and boron) than those from Uyuni. The magnesium-to-lithium ratio, which is undesirable for lithium concentration, is 6:1 in Atacama and 19:1 in Uyuni.
- Evaporation rates and precipitation also differ significantly: Atacama has an annual evaporation rate of 3,200 mm and precipitation of 10–15 mm/year, while Uyuni has 1,500 mm/year evaporation and 200–500 mm/year rainfall. In other words, Uyuni experiences lower evaporation and much higher rainfall, which will considerably slow the evaporation process.
- In Atacama, the evaporation process that concentrates lithium from 0.15% to 6% (a fortyfold increase) takes between 12 and 18 months. It is therefore expected that, in Uyuni, the evaporation period will be significantly longer, particularly during periods of heavy rainfall, such as those recently experienced, which flooded the pilot plant’s evaporation ponds.
| Main lithium minerals | ||
| Mineral | % Li máx. | % Li commercial |
| Amblygonite | 4.73 | 3.7-4.2 |
| Eucryptite | 5.50 | 2.6-3.0 |
| Lepidolite | Variable | 1.4-1.9 |
| Petalite | 2.26 | 1.4-2.2 |
| Spodumene | 3.73 | 2.6-3.0 |
| Average lithium content in processed brine | ||||||||
| Location | % Li | % Na | % K | % Mg | % SO4 | % Cl | % B | Li/Mg |
| Bolivia: salar de Uyuni | 0.025 | 8.80 | 0.72 | 0.65 | 0.046 | 15.7 | 0.02 | 1/19 |
| Chile: salar de Atacama | 0.14 | 7.6 | 1.87 | 0.93 | 0.03 | 16 | 0.1 | 1/1.64 |
| Israel-Jordan: Dead Sea | 0.0015 | 3.21 | 0.60 | 3.33 | 1.18 | 17.32 | 0.003 | 1/2200 |
| USA: Great Salt Lake, Utah | 0.004 | 8.0 | 0.65 | 1.00 | 0.016 | 14.0 | 0.006 | 1/250 |
| Silver peak, NV | 0.023 | 6.2 | 0.53 | 0.033 | 0.20 | 10.06 | 0.008 | 1/1.5 |
As an example, until 1997, brines from the Salar de Atacama were used exclusively for the production of lithium carbonate. Beginning in 1998, lithium chloride was also incorporated into the production process.
The production of lithium carbonate from these brines can be summarized in two main stages:
- Solution concentration through solar evaporation ponds – The initial lithium content of Salar de Atacama brines is approximately 0.17% Li, which is concentrated to values in the range of 4.3% to 5.8% Li.
- Treatment of the concentrated brine at the chemical plant. For the production of lithium carbonate (Li₂CO₃) with a purity of 99.5%, the concentrated brines are purified and crystallized, followed by a carbonation process, subsequent precipitation, and finally drying of the crystals.
Crystallization in Lithium Refining Processes
Crystallization is fundamental to obtaining lithium in a solid, pure form from extracted solutions. Regardless of the source (evaporated brines, DLE processes, or recycled battery leachates), the final refining stages often involve evaporation and crystallization to produce high-purity lithium salts.
After initial brine concentration and impurity removal, lithium is commonly precipitated as lithium carbonate (Li₂CO₃) or converted to lithium hydroxide (LiOH), both requiring controlled crystallization steps. Vacuum evaporators and crystallizers are used to further concentrate lithium solutions and induce formation of solid lithium crystals with minimal impurities. This is essential to reach battery-grade specifications (often ≥99.5% purity).

A lithium refining train may include multiple crystallization stages:
- Concentration: Evaporative concentrators (often under vacuum) increase the lithium content of a solution to near saturation. This step can follow any upstream extraction. For example, spodumene leach solutions or DLE eluates might be concentrated via multi-effect evaporation before crystallization.
- Primary Crystallization: A crystallizer induces lithium salt crystals to form from the concentrated solution, often by cooling and/or further vacuum evaporation. For lithium carbonate, this can be done by reacting the solution with soda ash (Na₂CO₃) and crystallizing Li₂CO₃, whereas lithium hydroxide can crystallize upon concentrating a LiOH solution. The product from this stage is typically technical grade (adequate purity for industrial use).
- Recrystallization/Purification: To achieve ultra-high purities needed for batteries, the technical-grade crystals may be re-dissolved and recrystallized to remove trace impurities. Multiple crystallization passes and sometimes ion-exchange polishing are used to reach 99.9% purity. This multi-stage crystallization approach allows lithium refiners to incrementally improve product quality. Notably, byproduct salts (like sodium sulfate, Na₂SO₄) can also be crystallized out from mother liquors as saleable products, improving overall process economics.
Vacuum crystallization technology is particularly suited for lithium refining because:
- It operates at reduced pressure, lowering boiling points and enabling water removal at relatively low temperatures.
- Gentle evaporation minimizes heat decomposition of lithium salts and can produce larger, high-purity crystals.
- It also permits the recovery of water and heat integration, aligning with sustainability goals.
Improvements in vacuum crystallization technology
Crystallization systems have seen significant innovation to meet efficiency and sustainability targets. Mechanical vapor recompression (MVR) and multi-effect evaporators are now commonly employed to reduce energy consumption.
- In MVR-based crystallizers, the water vapor boiled off is compressed and recycled as the heat source for further evaporation, drastically cutting external energy input. MVR lithium crystallizers can evaporate water with as little as ~40–60 kWh of electricity per ton of water, with no additional thermal energy required.
- Multiple effect systems represent a major improvement over single-effect evaporators. These systems often run under deep vacuum (e.g. 40–60% vacuum) to lower boiling points to 50–70 °C, allowing waste heat or low-grade renewable heat to drive the process.
Other engineering improvements include:
- Anti-scaling designs, such as forced circulation evaporators that reduce salt deposition on heat exchangers.
- Automated controls that optimize crystallization conditions and can modulate vacuum levels and flow rates to maintain optimal supersaturation, and perform self-cleaning cycles to remove scale.
- Performance improvements that boost uptime and ensure consistent product quality.
- Membrane‑assist: Nanofiltration pretreatment to knock down Mg/Ca, enabling higher concentration factors before crystallization.
- Data & compliance: KPI dashboards (water intensity, kWh/kg Li, recovery%) and auditable trails for ESG.
Research and development continue to focus on higher efficiency and integration: using renewable energy, capturing waste heat, and tightly integrating with upstream processes.
In fact, recent industry efforts demonstrate that pairing vacuum evaporation/crystallization with renewable power can make lithium refining nearly carbon-neutral.
Designed for ESG compliance
- High‑recovery evaporators + condensate polishing enable near‑closed‑loop water use in arid salars.
- Multi‑effect/MVR cut thermal demand; electrified evaporation eases renewables integration and decarbonization.
- Co‑product crystallizers (Na₂SO₄, KCl, gypsum) reduce tailings; solid residues are characterized and managed under best practices.
- Compact plants vs. evaporation ponds reduce land disturbance; enclosed operations limit air/soil impacts and simplify monitoring.
Pre-treatment with membrane separation
Membrane technologies (like nanofiltration and electrodialysis) are gaining attention to refine lithium streams. Nanofiltration (NF) can filter out divalent ions (Ca²⁺, Mg²⁺) from brines, which helps prevent scale and improves lithium purity downstream. In fact, researchers have used NF to remove magnesium from salt lake brines, significantly enhancing subsequent lithium recovery
Electrodialysis and special lithium-selective membranes can directly produce lithium hydroxide from lithium chloride solutions by electrically driving ions. However, membrane processes alone typically produce dilute lithium solutions or require further concentration. They are highly efficient at separating ions (often >95% purity in permeate or concentrate) but become less efficient at very high lithium concentrations
Therefore, membranes are excellent for upstream concentration/purification, while crystallization handles the final jump to a dry product. One notable case is the use of electrodialysis at a lithium plant to convert lithium carbonate into lithium hydroxide, followed by crystallizing LiOH·H₂O crystals out of the electrodialysis output (this avoids handling of hazardous chemicals like lime).
Strategic benefits for industry stakeholders
Investing in crystallization plants and complementary refining technologies offers several key advantages for lithium producers and project developer:
- Crystallization allows producers to hit the most demanding specs for battery-grade Li₂CO₃ and LiOH·H₂O (low impurities, controlled particle size). This opens access to premium markets and long-term supply contracts. Companies that master crystallizer operation can differentiate by offering reliably pure products, which is critical as impurities like Mg or boron can cause battery performance issues.
- Recovering more lithium from each ton of resource is an economic and sustainability win. Crystallization-centric flowsheets achieve higher overall yields thanks to the integration of different steps (reusing mother liquors, capturing byproducts, recycling water). Lithium recovery rates above 90–95% are reported after refining via crystallization, compared to maybe ~50% in some older evaporation operations. Higher efficiency means more lithium output from the same resource, reducing the need for new mines or brine wells.
- Adopting vacuum crystallization directly addresses many ESG concerns. Lower water consumption, reduced chemical waste, and the possibility of powered-by-renewables operations help projects earn a “green” or “sustainable lithium” label, something increasingly valued by investors and regulators.
- Crystallization technology is versatile. It can treat lithium from various sources (high-sulfate brines, geothermal fluids, recycled batteries, clays) and produce the desired output. Companies can pivot or diversify feedstocks without completely overhauling downstream equipment.
- As mentioned, crystallizers enable recovery of byproducts like potash (KCl), borates, or gypsum depending on the stream, which can be sold or reused. This not provides extra revenue, while moving the operation toward a circular economy model.
Industry Case Studies and Trends
Projects around the world are validating the benefits of vacuum crystallization in lithium production.
- Standard Lithium (Arkansas, USA) has been piloting a DLE-based lithium extraction from Smackover brines, followed by conversion to lithium hydroxide. In 2023 they reported their demonstration plant successfully extracted lithium via DLE and crystallized it into battery-quality lithium hydroxide (LiOH·H₂O). This process that turns brine into LiOH crystals, is one of the first of its kind in North America. It proves that integrating DLE with a crystallization/refining loop can produce high-purity lithium hydroxide suitable for EV batteries.
- AMG Lithium (Germany) is Europe’s first lithium hydroxide refinery, AMG Lithium GmbH is deploying evaporation and crystallization technology to produce battery-grade LiOH. The plant’s first module (20,000 tons per year LiOH) uses technical-grade lithium feed that is purified and crystallized into ultra-high-purity lithium hydroxide. The system includes pre-concentration, vacuum crystallizers, and dryers to ensure the final monohydrate crystals meet strict quality specs
- Covalent Lithium (Western Australia) new refinery leverages a conventional conversion of spodumene into lithium hydroxide, but with modern refinements. By early 2023, it was reported to be operating above 90% of nameplate capacity and producing on-spec battery-grade LiOH. While specific technical details are proprietary, the success is attributed to robust crystallization and purification stages that ensure consistency of product. This case underlines that even for mineral-sourced lithium, vacuum crystallization is key to achieving high yields and quality in new refineries.
- Chinese manufacturers have also invested in advanced evaporator-crystallizer systems for lithium extraction. For example, Myande Group advertises large-scale evaporator/crystallizer units for lithium recovery and recycling plants, claiming experience with leading battery and EV companies. These systems often integrate with chemical precipitation units and membrane systems. China’s brine operations (in Qinghai, etc.) face harsh climate and high magnesium content, so vacuum crystallization combined with pretreatment is used to produce lithium carbonate despite those challenges. Some Chinese projects recover lithium, then crystallize byproducts like magnesium chloride or borax, achieving near-total resource utilization.
- Vacuum evaporation and crystallization have proven extremely effective in recovering lithium from spent batteries. Companies in this space first leach or dissolve battery materials into solution, then use evaporation/crystallization to selectively pull out lithium salts (along with cobalt, nickel, etc.). For example, lithium in black mass leachate can be crystallized as lithium sulfate or carbonate and then reused in new battery production. By enabling high-purity recovery of lithium from waste, crystallization technology directly supports sustainability and supply security goals in the battery industry.
Our evaporation and crystallization systems for lithium refining
We supply tailored evaporation–crystallization trains that convert Li‑bearing solutions into battery‑grade Li₂CO₃ or LiOH·H₂O with high yield and low OPEX. Our evaporation and crystallization systems to assist lithium concentration and refining are:
- Solution 1. Concentration of the Li2SO4 solution
- Solution 2. Purification of Li2CO3
- Solution 3. Na2SO4 recovery as a byproduct during Li2CO3 production.
- Solution 4. LiOH production by reaction of Li2CO3 with lime
- Solution 5. LiOH production by electrodialysis
- Solution 6. LiCl production
- Solution 7. Water contaminated (tailings) treatment.

Solution 1. Concentration of the Li2SO4 solution
The sulfuric acid process for lithium extraction normally involves:
- Heating process at 1100ºC in a rotary kiln (α-spodumene is converted to β-spodumene)
- β-spodumene is grounded, mixed and roasted with H2SO4 at 250ºC.
- At this stage, Li2SO4 (soluble in water) is generated and an insoluble ore residue
- The excess of H2SO4 is neutralized with CaCO3. The kiln is then leached with water
- Filtration for impurities removal
- Lime addition to precipitate magnesium and soda ash to precipitate residual calcium
- pH adjustment with H2SO4, and Li2SO4 solution concentration by multiple-e ffect evaporation (triple effect) [1]. A highly concentrated lithium-containing solution is required
- Eventually, lithium can be precipitated as Li2CO3 by soda ash addition. Following crystallization, technical grade lithium carbonate is produced.
The use of multiple-effect evaporation can assist the lithium concentration process before lithium carbonate precipitation
- Multi‑effect/MVR evaporation concentrates liquor to target supersaturation while anti‑scaling dosing and seed recycle control fouling.
- Polishing precipitation (Mg/Ca removal), then Na₂CO₃ addition to precipitate Li₂CO₃ (technical grade).
- Recrystallization under vacuum yields battery‑grade Li₂CO₃; mother‑liquor goes to Na₂SO₄ recovery (Solution 3).
Solution 2. Purification/recrystallization of Li₂CO₃
- Re‑dissolution of technical grade; removal of B, Mg, Ca, Na, K.
- Controlled cooling + evaporative recrystallization to achieve ≥99.5–99.9% purity and target particle size.
Solution 3 : Na2SO4 recovery as a byproduct during lithium carbonate production
Main stages:
- Na2CO3 is added to a concentrated Li2SO4 solution in order to precipitatelithium carbonate
- By evaporation and crystallization, lithium carbonate is obtained
- A residual solution containing sodium sulfate can be eventually concentrated in acrystallizer for Na2SO4 (by-product) recovery.
Optional integration with ZLD, minimizing liquid waste and monetizing salts. The use of evaporation-crystallization technology can facilitate sodium sulfate recovery (by-product).
Solution 4. LiOH production by reaction of Li2CO3 with Ca(OH)2
- Li2CO3 + Ca(OH)2 –> 2LiOH + CaCO3
- A monohydrate LiOH can be produced by evaporation-crystallization.
- The use of crystallization technologies can assist in LiOH production from Li2CO3.
Post‑reaction clarification;
- Vacuum crystallization of LiOH·H₂O.
- mother‑liquor recycle.
- Solid CaCO₃ filtered for sale or safe disposal.
Solution 5. LiOH production by electrodialysis
During electrodialysis (ED) treatment of a Li2SO4 solution, the cathode chamber is enriched with LiOH while the anode one is enriched in H2SO4.
However, other alkali metals like sodium and potassium will also be enriched in the cathode chamber. This could be solved by implementing a crystallization process following ED.
NaOH and KOH solubility is 10 times higher than LiOH solubility. Thanks to selective crystallization, it is feasible to precipitate most LiOH as higg-purity crystals, while keeping NaOH and KOH in the solution.
Solution 6. LiCl production
In modern flowsheets, LiCl appears as an intermediate from DLE eluates (chloride media), high‑chloride brines, or from converting Li₂CO₃/Li₂SO₄ streams. Reaching battery‑grade salts still depends on impurity control + vacuum crystallization.
Generation & direct production:
- From Li₂CO₃ or LiOH using HCl; polish Ca/SO₄ as required (e.g., oxalate/barium).
- Electrodialysis (ED) can convert LiCl directly to LiOH; subsequent vacuum crystallization of LiOH·H₂O captures lithium while Na/K largely remain dissolved due to their higher solubility.
LiCl in modern refining (four key steps)
- Impurity polishing. Reduce Mg²⁺/Ca²⁺ (lime/caustic or NF), remove boron (solvent extraction/IX), and condition sulfate/chloride to the target route.
- Route selection.
- LiCl → Li₂CO₃: Carbonate addition (Na₂CO₃) to precipitate technical Li₂CO₃, then re‑dissolve + recrystallize under vacuum to ≥99.5–99.9% and controlled PSD.
- LiCl → LiOH: ED or lime conversion to LiOH, followed by vacuum crystallization of LiOH·H₂O.
- Crystallization & washing. Because LiCl is highly soluble while Li₂CO₃ is sparingly soluble, crystal washing and counter‑current cold washes are critical to push residual Na/K/Mg below spec with high yield.
- Mother‑liquor management. Recycle liquors to upstream polishing or crystallize by‑products (Na₂SO₄/KCl/gypsum) to reduce waste and improve economics (ZLD‑ready).
Solution 7. Tailings treatment / ZLD
A ZLD solution is targeted with a combination of water and solid waste treatment technologies:
- Water recovery.
- Metals recovery.
- Reduced effluent volume.
The application of membrane and evaporation-based concentration technologies facilitate a ZLD solution.
Condensate reuse (boiler/utility), crystallized solids managed as stable, classified salts; options for road‑base or construction fillers when compliant.
Real examples for the precipitation and refining of lithium chloride
Example 1. Process for Li-rich brine in Atacama, Chile
Lithium carbonate is the most important base compound among lithium salts, accounting for approximately 60% of total lithium product demand. Its importance lies mainly in its ease of purification and its suitability for conversion into other inorganic and organic lithium salts, such as lithium cobalt oxide (LiCo), lithium bromide (LiBr), lithium hydroxide monohydrate (LiOH·H₂O), and other compounds.
Global production is estimated at 45,000 metric tons per year, with Chile as the leading producer, while the United States is the largest consumer of lithium carbonate.

Lithium brine extraction from the Salar de Atacama has been carried out by Sociedad Chilena del Litio (SCL) since 1984. The company operates a plant with an annual production capacity of 11,800 metric tons of lithium carbonate (Li₂CO₃).
- Brine extraction from the salt flat is carried out using pumps that draw the brine from a depth of 30 meters, transporting it through pipelines to a system of solar evaporation ponds where the lithium concentration increases from 0.17% to 4.3%. The construction of the ponds within the salt flat involved breaking the salt crust to create a flat surface over a clay layer. Both the dikes and the bottoms of the evaporation ponds were lined with a durable 0.5 mm plastic membrane.
- The polyurethane lining is protected with a layer of sodium chloride (NaCl) salts approximately 30 cm thick. During evaporation, salts precipitate sequentially in the ponds and are harvested and discarded as impurities: halite (NaCl), sylvinite (NaCl + KCl), carnallite (KCl·MgCl₂·6H₂O), and bischofite (MgCl₂·6H₂O).
- In the ponds with the highest concentrations, lithium carnallite (LiCl·MgCl₂·7H₂O) precipitates. To recover the lithium contained in it, the carnallite is repulped and washed with a solution saturated in magnesium chloride but not saturated in lithium chloride.
- Any bischofite (MgCl₂·6H₂O) present and undissolved is removed by centrifugation and eliminated from the system. The brine, now concentrated to 5.8% Li, 20% Mg, and 0.7% B (expressed as boric acid, H₃BO₃), is then ready to be transported to the chemical plant located in Antofagasta, 170 km from the Salar de Atacama.
- At the La Negra plant, chemical treatment consists of removing the remaining magnesium in two purification stages: first as magnesium carbonate, then as magnesium hydroxide.
- For this purpose, the concentrated brine is diluted to 0.6% Li using mother liquor from the final stage of lithium carbonate precipitation.
- The product is obtained through a hot reaction (approximately 85 °C) between the magnesium-free purified brine (1 ppm) and a sodium carbonate (Na₂CO₃) solution, precipitating lithium carbonate (Li₂CO₃).
- The final product is dried and marketed either as crystals (70%) or compacted granules (30%).
The product purity is approximately 99.5% Li₂CO₃. However, its boron content (400–600 ppm) prevents its use as a raw material for producing metallic lithium via lithium chloride.
2LiCl + Na₂CO₃ → Li₂CO₃ + 2NaCl
To address the boron issue and achieve lithium carbonate that meets the highest market specifications, Foote designed a process that removes boron from the brine through solvent extraction before the remaining magnesium is separated.
In this process, the liquid–liquid extraction unit first removes the residual boron from the concentrated brine sourced from the salt flat. The process then proceeds as described above, ultimately producing lithium carbonate with very low boron content (less than 5 ppm).
In an initial stage, most of the magnesium is leached out, leading to the formation of a lithium chloride solution with low sulfate content. This solution is then treated with sodium carbonate to obtain lithium carbonate.
In 1998, MINSAL estimated production at 9,000 metric tons of Li₂CO₃, with capacity exceeding 20,000 metric tons per year. The company has considered expanding by building a butyllithium plant in Texas to support battery material production.
The process developed by MINSAL differs significantly from SCL’s in its initial stages, as it uses harvested salts from ponds containing lithium sulfate as its raw material.
In 1997, SQM, leader in the nitrate market, began producing and marketing lithium carbonate from the brines of the Salar de Atacama.
A fraction of the brine resulting from the solar evaporation process for potassium chloride production continues its concentration process and becomes the source of lithium for SQM’s lithium carbonate production at its plant located in Salar del Carmen.
The lithium-concentrated brine is transported by truck from the Salar de Atacama to the plant, where it is first purified to remove residual boron and then magnesium through extraction and filtration processes.
Finally, the purified lithium brine reacts with sodium carbonate to produce lithium carbonate, which is then filtered, washed, dried, and packaged in various formats—ranging from fine products for use in lithium-ion rechargeable battery manufacturing to granular products used in aluminum production.
Both SQM and FMC (SCL) transport concentrated brines from the salt flat to their plants in Antofagasta, effectively supplying most of the American market (88% of U.S. lithium imports come from Chile, 7.5% from Argentina, and the remainder from small quantities imported from China and Japan).
Lithium chloride can be obtained from lithium carbonate or lithium hydroxide by reacting with hydrochloric acid:
Li₂CO₃ + 2HCl → 2LiCl + H₂O + CO₂
LiOH·H₂O + HCl → LiCl + 2H₂O
- To remove sulfate and calcium present in the lithium carbonate brine, oxalic acid and barium chloride must be added to the reactor.
- The brine is then filtered to remove impurities.
- The process then proceeds to crystallization, centrifugation, and drying. The crystallized lithium chloride is washed with cooled water in a countercurrent flow and then sieved.
- From LiCl, metallic lithium can be obtained, which is used in Li–Al alloys and primary batteries (energy sources).
One of the advantages of this element is that it can be heated to 600 °C without decomposing. When heated to 800 °C in a hydrogen atmosphere, it partially decomposes into lithium oxide and carbon dioxide—a compound with low solubility.
LiCI CRYSTALLIZATION PROCESS
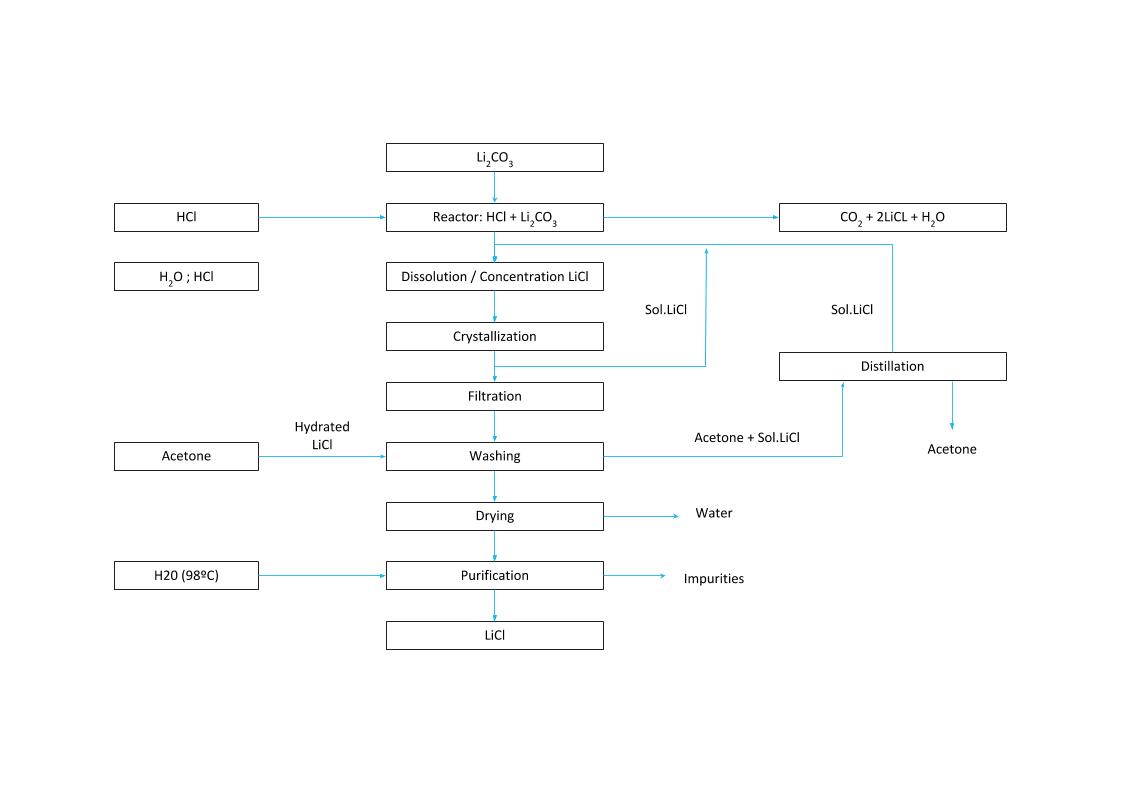
Chemical process without natural evaporation
LITHIUM CRYSTALLIZATION PROCESS (2)
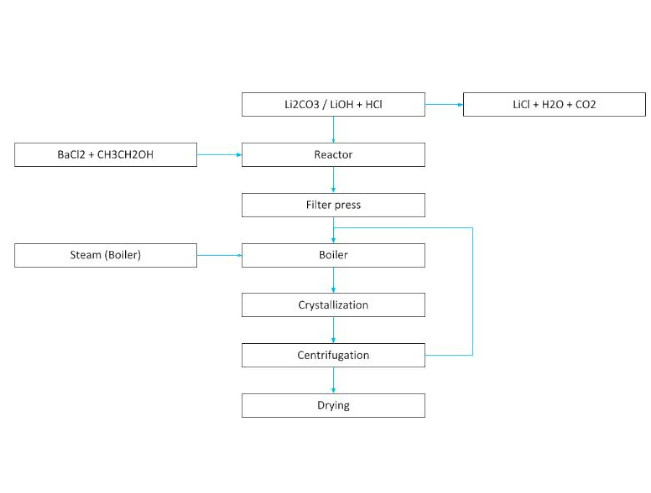
Example 2. Differential process of obtaining LiCl
In a sequence of evaporation ponds arranged in series, as shown in the previous diagram, sodium chloride, sodium–potassium chloride, and potassium–magnesium chloride precipitate due to differences in solubility.
Once the brine reaches an approximate concentration of 6% LiCl, lime is added to precipitate magnesium, and boron can be removed as an isopropyl alcohol ester through solvent extraction, with the solvent recovered by distillation.
D. Galli has described these processes in detail in the patent held by ADI, related to the exploitation of the Rincón salt flat in Salta, Argentina.
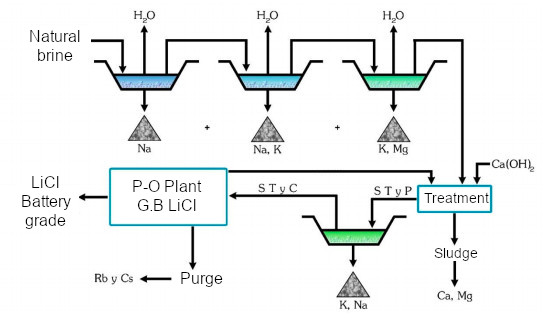
Pond scheme in the evaporation process. Source: Dr D. Galli.
After the addition of lime, Mg, Ca, and B are removed, and the process can follow different routes depending on the desired lithium compound:
- By adding Solvay soda (sodium carbonate), an impure lithium carbonate is precipitated, which is then converted into lithium bicarbonate by CO₂ injection. This bicarbonate is filtered and heated to obtain battery-grade Li₂CO₃.
- Alternatively, by electrodialysis of the concentrated lithium chloride solution, high-purity LiOH·H₂O and LiCl can be obtained. The company Simbol has patented a method for purifying LiOH via electrodialysis of LiCl to achieve battery grade.
- Another alternative is to treat the brine in ion-exchange resin columns that remove contaminants before adding Solvay soda to produce high-purity lithium carbonate. It should be noted that elution and regeneration of the columns generates large volumes of liquid waste.
The soda–lime process allows the removal of Mg²⁺ and SO₄²⁻ by precipitation as magnesium and calcium sulfates, which form contaminated sludge. However, these can be used to stabilize roads during salt flat development or as fire-resistant filler material for construction—considered a strategic natural resource.
Boron must be removed because of its negative impact on metallic lithium production, for which solvent extraction using isopropyl alcohol is applied, forming boron esters.
With the soda–lime method, technical-grade Li₂CO₃ (>99.5%) can be obtained. This product can be re-dissolved as soluble lithium bicarbonate (LiHCO₃) by bubbling CO₂, filtered, and then, by increasing temperature, the CO₂ is removed and battery-grade lithium carbonate (>99.9%) is precipitated, with CO₂ recycled.
This process could also be implemented as a method of atmospheric carbon dioxide capture, generating potential benefits from carbon credits. Lithium chloride can be brought to high purity by re-dissolution in isopropanol, which must be distilled to recover the solvent.
Lithium carbonate can also be dissolved in HCl and treated in ion-exchange columns to obtain high-purity LiCl. Finally, metallic lithium is obtained by electrolysis of a molten eutectic mixture of KCl–LiCl at about 400 °C under an argon atmosphere.
Since using solvents more expensive than water is costly, they must be recovered by distillation, which increases energy costs, so their impact on the final product cost must be carefully evaluated.
Salt flats with high magnesium content, such as Atacama and Uyuni, pose problems due to the flocculation of magnesium hydroxide during lime precipitation. In such cases, initial magnesium removal by precipitation with Ca(OH)₂ before the solar evaporation stage is recommended. For high-magnesium salt flats, the sulfate route is preferred over the chloride route.
Example 3. Selective lithium recovery (LiOH)
The selective recovery of lithium from brines containing less than 1% Li in the presence of high concentrations of other alkali and alkaline earth ions is an industrial objective.
Evaporative processes are based on the differential solubility of lithium salts in concentrated brine solutions, i.e., fractional recrystallization.
Alternatively, selective chemical and electrochemical processes have been designed to recover high-purity lithium chloride, hydroxide, or carbonate, aiming to reduce processing times and minimize environmental impact from water loss and the generation of harmful waste.
A recently proposed fast method is based on the precipitation of sparingly soluble lithium phosphate (Li₃PO₄, solubility 0.39 g/L) by treating brine with phosphoric acid; the insoluble lithium phosphate is then treated with lime to form highly insoluble hydroxyapatite, while soluble lithium hydroxide is recovered:
3Li₃PO₄ + 5Ca(OH)₂ → Ca₅(PO₄)₃OH + 9LiOH
In lithium extraction processes from Argentine salt flats, the phosphoric acid is recovered by treating hydroxyapatite with sulfuric acid, forming hydrated calcium sulfate (gypsum), which has applications in construction:
Ca₅(PO₄)₃OH + 5H₂SO₄ → 5CaSO₄·2H₂O + H₃PO₄
This method was patented by the Korean steel company Posco, which built a pilot plant in Cauchari, Jujuy, in 2015.
The method does not require brine evaporation, making it significantly faster than evaporative methods. However, because it uses phosphoric acid—which, although recovered, may leave magnesium and calcium phosphate residues as contaminating sludge, waste management is necessary.
Adsorption methods
1. The selective adsorption of lithium from brines (300–1000 ppm) and seawater (0.125 ppm) has been extensively studied with adsorbents such as MnO₂, TiO₂, and Al(OH)₃.
- Lithium uptake depends on intercalation into non-stoichiometric oxide lattices.
- Capacity ranges: 3–35 mg/g depending on adsorbent.
- Extraction from lithium-rich brines (>5 mg/L): >20 mg/g achievable.
- Limitation: possible co-insertion of Mg²⁺, Na⁺, K⁺, Ca²⁺.
2. Manganese oxide has been studied as an adsorbent in various forms, such as MnOx ion sieves, with subsequent lithium recovery by acid leaching to yield, for example, Li₀.₁₅H₀.₇₆Mg₀.₄₀MnIII₀.₀₈MnIV₁.₅₉O₄.
- Cubic spinel λ-MnO₂ can incorporate 38 mg/g lithium → LiMn₂O₄.
- Tested in Uyuni (Bolivia) by Korean researchers.
- Problem: insufficient oxide stability in leaching columns at industrial scale. During acid treatment, which replaces lithium ions with protons in the crystal structure during elution, the mixed oxide dissolves, releasing other ions such as Ca²⁺ and Mg²⁺.

3. Interest has also been given to natural minerals that can capture lithium in the Earth’s crust, as model systems for lithium adsorption and absorption.
For example, gibbsite, an aluminum hydroxide mineral, has been studied in detail for lithium uptake. Various companies, including Dow Chemical Co., FMC (Foote Mineral Company), Simbol Inc., and Posco, have patented methods for lithium recovery using amorphous aluminum hydroxide in various forms.
4. The international mining corporation FMC, operating in Argentina’s Salar del Hombre Muerto (Catamarca) through its subsidiary Minera del Altiplano S.A., uses proprietary technology based on ion exchange with zeolites (likely gibbsite-type) controlled by temperature. The process goes as it follows:
- Lithium ions are extracted from concentrated brines containing LiCl (after pre-concentration to 9 g/L, usually by solar evaporation).
- The liquid is passed through a column of polycrystalline hydrated aluminum hydroxide supported on agglomerated material until lithium saturation is reached.
- In the following stage, LiCl is displaced from the ion exchanger using concentrated NaCl solution repeatedly, followed by a diluted LiOH solution.
5. Ion-exchange resins such as Zeo-Karb 225, Dia-ion, SK, and AG50WX8, containing sulfonate groups and chelating agents, have also been used to capture lithium from synthetic brines.
6. Organic solvent extraction of lithium complexed with organic agents has been proposed as well. Lithium complexed with organic agents. These methos presents the following challenges:
- High resin/solvent cost.
- Energy requirements for regeneration.
- Potential environmental impact of effluents.
Electrochemical methods
Electromechanical methods are widely used to extract lithium from brines, as they present significant benefits:
- Minimize water loss through evaporation.
- Avoid environmental contamination from chemical residues such as NaCl or MgSO₄.
- Remain cost-effective.
1. Kanoh reported lithium ion intercalation into λ-MnO₂ cathodes using an electrochemical cell with a platinum anode, studying the λ-MnO₂/LiMn₂O₄ ion insertion/extraction kinetics in LiCl solutions. The drawback of this cell is anode reactions that alter brine pH through water decomposition.
2. La Mantia and collaborators used entropic cells to extract lithium with LiFePO₄ cathodes and Ag/AgCl anodes—without pH changes—but with high silver cost and dissolution in highly concentrated chloride solutions.
More recently, these authors introduced a nickel hexacyanoferrate anode that exchanges cations as an alternative to Ag/AgCl. Lee reported a similar electrochemical cell combining λ-MnO₂ with a silver anode for lithium extraction from artificial brines.
3. Kim used the same manganese oxide cathode combined with a carbon capacitive electrode in a supercapacitor configuration. These configurations have been reviewed by Missoni. Highly lithium-selective methods over sodium employ electrochemical processes using olivine-structured LiFePO₄ cathodes coated with dopamine and I⁻/I₃⁻ systems.
4. Hoshino proposed electrodialysis with an ionic liquid membrane, although with very low extraction rates.
5. Liu proposed a method using two electrodes—LiFePO₄ and FePO₄, separated by an anion-permeable membrane for lithium extraction from brines. The process follows two main stages:
- Lithium ions from LiFePO₄ combine with anions (X⁻), increasing LiX concentration.
- Lithium ions intercalate into FePO₄ in the other compartment, decreasing LiX concentration.
6. In Argentina, researchers at INQUIMAE have developed an alternative electrochemical lithium extraction method from natural Puna brines, patented by CONICET. This method has some advantageous characteristics:
- Fast
- Environmentally friendly (no chemical additives or waste generation)
- Low in energy consumption
- Highly selective for LiCl extraction.
The proof of concept has been completed, and reactor engineering and scale-up are underway. The brine circulates through an undivided electrochemical cell using a Li₁₋ₓMn₂O₄ battery-type cathode (0 ≤ x ≤ 1) that selectively captures Li⁺ by intercalation, and polypyrrole (PPy) as an anode that selectively captures Cl⁻ through charge compensation during oxidation.
The process goes as it follows:
- First, the brine is exposed to reduced Li₁₋ₓMn₂O₄ and oxidized PPy electrodes, spontaneously capturing LiCl while generating energy.
- After rinsing the electrodes, the brine is replaced with a dilute electrolyte, and the cell polarity is reversed, releasing LiCl into the solution. Under a potential difference of less than 1 V, Li⁺ ions intercalate into Li₁₋ₓMn₂O₄ and Cl⁻ ions adsorb onto oxidized PPy. The energy needed for this second step, and for pumping and circulation, can be supplied by solar panels, as the Puna region receives over 2,600 kWh/m² of annual solar radiation. It has been estimated that capital investment in solar panels with a 30-year lifespan amounts to just $10 per ton of lithium chloride extracted.
- During LiCl capture, only Li⁺ ions intercalate selectively into manganese oxide in contact with highly concentrated brine containing sodium, potassium, magnesium, etc.
- LiMn₂O₄ is a stable phase with half the lithium content in the discharge from λ-MnO₂ to Li₂Mn₂O₄. It has a cubic spinel structure (space group Fd3m) with a unit cell containing 56 atoms: an oxygen-ion-packed lattice with 16 Mn in octahedral sites (MnO₆) and 6 lithium ions in tetrahedral 8a sites.
- Lithium ion insertion/extraction occurs via a topotactic process within the cubic structure, with isotropic expansion as revealed by X-ray diffraction peak shifts.
- By using a chloride-selective electrode, it is possible to extract lithium chloride from brines with high selectivity by adjusting the Mn³⁺/Mn⁴⁺ redox potential in the crystal structure.
- Because there are two non-equivalent tetrahedral sites for Li⁺ in the spinel, two redox processes are observed in this positive electrode material for batteries.
The lithium chloride extraction process delivers excellent results:
- Highly selective and efficient within the LiMn₂O₄/λ-MnO₂ stoichiometry,
- High reproducibility over more than 200 charge–discharge cycles,
- Low water consumption,
- Low energy use (5 Wh/mol based on charge and 10 Wh/mol based on recovered lithium concentration).
- No sodium or magnesium co-insertion into manganese oxide has been observed by X-ray diffraction evidence.
Engineering design and scale-up of electrochemical reactors for lithium extraction from natural brines using this method are currently underway.
LiMn₂O₄ has a lithium uptake capacity of 38 mg/g. As the lightest metal, lithium can store a large amount of charge per unit mass. However, when recovered electrochemically, a large charge is required: every 7 g of lithium requires 1 Faraday (26.8 Ah), giving rise to the so-called “lithium paradox” in lithium extraction from Argentine salt flats—making careful design of reactors with high-surface-area three-dimensional electrodes a key factor.
Bibliography
- O. A. Hougen, K.M. Watson, R. A. Ragatz, Principios de los Procesos Químicos. Parte II Termodinámica, Editorial Reverté, Madrid, 1964.
- O. A. Hougen, K.M. Watson, R. A. Ragatz, Principios de los Procesos Químicos. Parte I. Balances de Materia y Energía, Editorial Reverté, Madrid, 1964.
- J. M. Smith, H. C. Van Ness, Introducción a la Termodinámica en Ingeniería Química, 3ª Edición, Editorial McGraw-Hill, 1982.CAP.1. INTRODUCCIÓN 9
- Roine, A., HSC Chemistry Software, Versión 5.11, Outokumpu Research Oy, Información Servie P. O. Box 60 FIN-28101 PORI, Finland, 2005.
- L. David, Parkhurst, C. A. J. Appelo, User’s Guide to PHREEQC (version 2), U. S. Geolical Survey Box 25046, MS 418, Denver, Colorado, 1999.
- gPROMS ModelBuilder version 2.3.1, Process Systems Enterprise Limited, 2004.
- K. S. Pitzer, J. Phys. Chem., 77(1973) 268-277.
- H. Renon, J. M. Prausnitz, AIChe J., 14 (1968) 135-144.
- C.F. Weber, Eng. Chem. Data, 39 (2000) 4422-4426.
- Y. Li, P. Song, S. Xia, S. Gao, CALPHAD, 24 (2000) 295-308.
- F. Farelo, C. Fernandes, A. Avelino, J. Chem. Eng. Data, 50(2005) 1470-1477.
- C. Monnin, M. Dubois, N. Papaiconomou, J. P. Simonin, J. Chem. Eng. Data, 47 (2002) 1331-1336.
- Chr. Christov, Chr. Balarew, S. Petrenko, Vl. Valyashko, Journal of Solution Chemistry, 23 (1994) 595-604.
- Z. Li, T. Deng, M. Liao, Fluid Phase Equilibria, 293 (2010) 42-46.
- Chr. Christov, Computer Coupling of Phase Diagrams and Thermochemistry, 36 (2012) 71-81.
- D. Zeng, Z. Wu, Y. Yao, H. Han, J. Solution Chem, 39 (2010) 1360-1376.
- J. M. Prausnitz, R. N. Lichtenthaler, E. G. Azevedo, Molecular Thermodynamic of Fluid-Phase Equilibria, Prentice-Hall, Inc, Englewood Cliffs, NJ, 1998.
- D. A. Weingaertner, S. Lynn, D. N. Hanson, Ind. Eng. Chem. Res., 30 (1991) 490-501.
- T. A. Graber, H. Medina, H. R. Galleguillos, M. E. Taboada, J. Chem. Eng. Data, 52 (2007) 1262-1267.
- Y. T. Wu, D. Q. Lin, Z. Q. Zhu, L. H. Mei, Fluid Phase Equilibria, 124 (1996) 67-69.
- Vera et al. Nature Reviews Earth & Environment (2023) – Environmental analysis of traditional vs. direct lithium extraction
- Sterlitech (2024) – Overview of lithium extraction methods and innovations
- IEA Global Critical Minerals Outlook (2024) – Lithium demand growth and ESG risk data
- Wastewater Digest (Feb 2025) – on lithium battery recycling via vacuum evaporation & crystallization
- Saltworks Tech. – SaltMaker MVR crystallizer spec sheet, energy consumption data
- Standard Lithium (Aug 2023) – Press release on DLE pilot producing battery-quality LiOH
- AMG Lithium/GEA (2021) – Announcement of LiOH plant using GEA crystallization tech
- Sterlitech (2024) – Lithium recovery part 2, circular economy and membrane use