Sections
- Introduction
- What are PFASs?
- How do PFASs affect the environment and human health?
- Treatment of effluent containing PFASs
- Vacuum evaporation: A PFAS treatment solution
- Summary
INTRODUCTION
PFASs are formed by a wide group of highly stable chemical products. These products have been manufactured and used in a wide variety of industries around the world since the 1940s.
They are found in a wide range of products that consumers use every day, such as cookware, pizza boxes and stain repellents. Most consumers have been exposed to these compounds for many years. Certain PFASs can accumulate and remain in the human body for a long time.
There is evidence that exposure to PFASs can cause detrimental health effects. The most studied PFASs are PFOA and PFOS.
Laboratory animal studies indicate that these chemicals can cause adverse effects on the reproductive and immune systems, as well as in development and organs such as the liver and kidney. Both chemicals have caused tumors in animals.
The clearest findings in people exposed to them are higher cholesterol levels.
In many chrome-plating industries in the United States, PFASs were introduced initially to protect the environment from chromium in fumes. However, PFASs were later found to be harmful to both the environment and human health.
Recent studies have shown alarming consequences of PFAS exposure, including a detrimental impact on growth and learning in children and increased cancer risks.
Many companies voluntarily gave up using PFASs in 2002, and this was followed globally by many more in 2015; since then, surface protection factories no longer use PFAS or PFOS.
However, there is a problem with contaminated surface and ground water that will need to be pumped and treated.These water companies have to comply with the strict PFAS limits in rainwater discharge and groundwater.
These apply across the board, nationwide in the US, with many states having limits that are even stricter.
What are PFAS?
A perfluoroalkylated substance (PFAS) is a chemically synthesized compound consisting of fluorinated, hydrophobic alkyl chains of variable length, with a hydrophilic end group.
Due to this amphiphilic character, these substances have great chemical and thermal stability, as well as high surface activity.
For all these reasons, PFASs are widely used in industrial and consumer applications that include anti-stain coatings for fabrics and carpets, paints and varnishes, furniture, shoes, lipophobic coatings for paper products suitable for food contact, extinguishing foams, surfactants for mining or oil extraction wells, soil brighteners and insecticides.
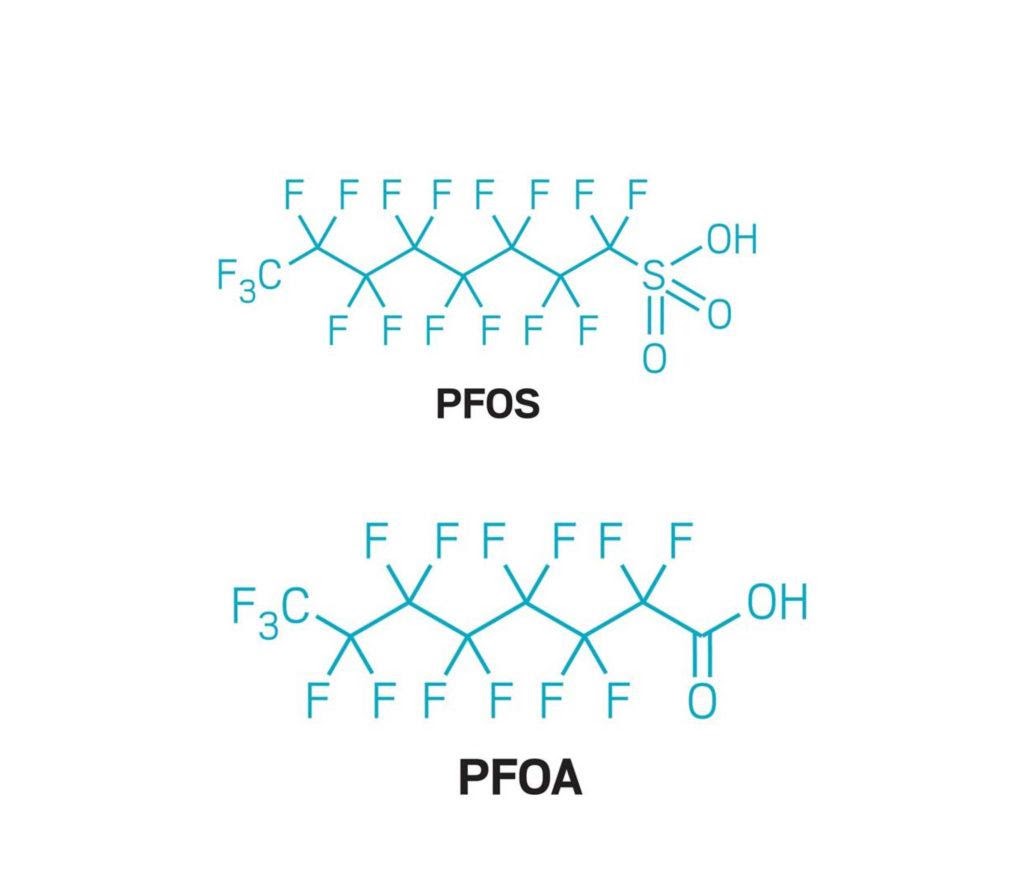
An important subgroup are perfluorinated organic surfactants, to which perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) belong.
Chemical structure:
There are many other PFASs in use within industry, such as GenX and PFBS.
GenX is the trademark for a technology used to make high-performance fluoropolymers (e.g. some non-stick coatings) to replace the use of perfluorooctanoic acid (PFOA).
Hexafluoropropylene oxide dimer acid (HFPO) and its ammonium salt are the main chemicals associated with GenX technology. GenX chemicals have been found in surface water, groundwater, drinking water, rainwater and air emissions in some areas.
How do PFASs affect the environment and human health?
PFASs have been manufactured and used in a wide variety of industries around the world. They have been used in the United States since the 1940s.
Of these chemicals, PFOA and PFOS have been the most produced and studied. Both are extremely persistent in the environment and in the human body; that is, they do not break down and can accumulate over time.
There is evidence that exposure to PFASs can cause detrimental health effects. PFASs can be found in:
- Food: In packaging using materials containing PFASs, when processed with equipment that used PFASs, or grown in soil or water contaminated with PFASs.
- Household products: Such as water and stain repellent fabrics, non-stick products (such as Teflon), polishing compounds, waxes, paints, cleaning products and firefighting foams (they are a major source of groundwater contamination in airports and military bases where firefighting training is carried out).
- Workplaces: Especially manufacturing plants or industries that use PFASs, such as chrome plating, electronics manufacturing and oil recovery.
- Drinking water: Commonly located and associated with a specific plant (e.g. manufacturers, landfill, sewage treatment plants and firefighter training centers).
- Living organisms: Such as fish, animals and humans, where PFASs can accumulate and persist over time.
Due to such widespread use, PFOS and PFOA, their salts and precursors have been detected in the environment, fish, birds and mammals.
PFASs have been manufactured for over 50 years in a wide variety of consumer products, as well as in agricultural applications, which has led to their dispersion throughout the environment, entering the food chain until they were included in Annex B of the Stockholm Convention in 2010 to restrict their use to a specific list of applications.
Although their production has been limited worldwide, their release into the environment is mainly caused by the contribution of products treated with PFASs, or by inappropriate disposal of products that contain them.
PFASs pose a health risk. Concern about their adverse effects on public health arose after several experimental studies in animals indicated these substances had toxicological repercussions: hepatotoxicity, negative effects on development and behavior, immunotoxicity, reproduction and lung damage, hormonal effects, as well as genotoxic and carcinogenic potential, although these results were not shown to have implications for human health.
According to the European Food Safety Authority (EFSA), diet is the main source of human exposure to PFASs, in particular fish, fishery and meat products (mainly liver). However, there are other non-food sources of exposure, such as air polluted with PFOA, which also contribute to total exposure.
There are other less important routes of exposure, such as PFOS and PFOA in process water, non-stick cookware and PFOA in food packaging materials (e.g. microwave popcorn bags).
The EFSA concluded in 2008 that the average population in Europe is unlikely to experience negative health effects from dietary exposure to these contaminants, with only some high fish consumers slightly exceeding the PFOS toxicological reference value.
Some of the PFASs were considered during the Stockholm Convention on Persistent Organic Pollutants (POPs) of 2010, which is the most ambitious instrument at an international level to regulate and control these substances to protect human health and the environment, signed in 2001.
The European Union and all its Member States signed the Convention and established Regulation 850/2004, April 29, 2004, on persistent organic pollutants to guarantee coherent and effective application of the obligations contracted under it at the European level.
In its 2008 scientific opinion on PFASs, the EFSA recommended collecting more data on these substances in foods to improve the accuracy of calculating future exposure through diet.
In this sense, the European Commission published Recommendation, 2010/161/EU, to monitor the presence of some of these substances in a wide variety of foods. In the latest EFSA report on PFASs in 2012, over 54,000 PFAS analytical results from 13 European countries (including Spain) were collected during the period 2006 to 2012.
Of the 27 substances included in the exposure assessment, the proportion of quantified results was very low, meaning that the levels of these contaminants found in food were very low. Thus, the EFSA confirmed the low health risk to the public from exposure to these substances in the diet.
Subsequently, in 2014, a scientific report on the oral toxicity of these compounds in animals and humans was published by the EFSA, due to the large number of perfluoroalkylated substances, their precursors and the substances derived from them.
This was a systematic review of the current scientific literature, to provide undoubted help to bodies assessing the risk of these compounds worldwide, such as the General Sub-Directorate for the Promotion of Food Safety. The EFSA established a tolerable daily intake (TDI) of 150 ng/kg body weight for PFOS and 1,500 ng/kg body weight for PFOA; these are the maximum daily amounts people can ingest during their lifetime without causing adverse health effects.
The European Commission recommends using EU harmonized sampling and analysis methods for dioxins and PCBS as a reference for the control of PFAS, established in the Commission Regulation (EU) 589/2014. The performance criteria for the analysis method of these substances are specified in Recommendation 2010/161/EU.
Treatment of effluent containing PFASs
Conventional wastewater treatment processes are effective for many PFASs by separating them into sludge, although this is a challenge as there is a wide variety of more than 3,000 individual compounds. Of these, only 24 are routinely measured.
It is not unusual for one or more of these compounds to have higher concentrations in a treated effluent than in the influent before treatment for PFASs.
This is because the treatment process reacts with some of the thousands of PFASs present to transform or degrade them into one of those which are subsequently quantified.
One strategy to address this treatment problem is to minimize the amount of PFASs entering the WWTP process. Research has been conducted in some states to identify and address the sources of PFASs.
Once identified, the procedure can be applied through the Industrial Pretreatment Permit Program (IPP) to require industries to reduce or eliminate these PFASs before discharging them into the sewage system.
These additional pretreatment requirements for industrial sources could have financial consequences for the community and operational implications for the WWTP, which means that this strategy must be carefully considered and supported with sampling data.
Another potential strategy is to employ additional treatment technology to remove PFASs before access.
To date, drinking water suppliers have used granular activated carbon (GAC) and reverse osmosis (RO) as the most effective treatment strategies, but both technologies are expensive to implement.
These solutions or some of their variants have also been tested in wastewater treatment. Clearly, these techniques will still leave the utility company with the problem of removing contaminated material, as these techniques are for separation only.
There are also destructive techniques, such as electrochemical oxidation and incineration, that break down the chemical structure of PFASs. However, most of these methods are at the research and development stage or small-scale pilot test phase and, in the case of incineration, are prohibitively expensive.
Presence in wastewater sludge
PFASs have been found in biological sewage sludge, much of which is processed and used on the land for agricultural use. Application on land is mutually beneficial: WWTPs have cost-effective methods of removing sludge, while the farmer enriches his soil with nutrients.
However, according to some research, the application of municipal sludge to the land may be a potential source of PFAS contamination in aquifers via percolation from these fields.
However, there are currently no regulations governing the levels of PFAS in biological sludge. Most countries are adopting controls on sludge from sewage treatment plants, starting with the collection of data on PFASs in biosolids (in Michigan and Maine, for example).
As noted above, the US EPA Action Plan and the House of Representatives bill include plans to classify PFASs as hazardous substances. This could greatly affect the ability to cost-effectively remove PFAS-containing biosolids via land application.
The National Association of Clean Water Agencies (NACWA), the Water Environment Federation (WEF) and the Water Research Foundation (WRF) are actively investigating the treatment of PFASs in wastewater and characterizing the potential risk to human health of using this sludge as agricultural fertilizer.
Protection of drinking water supplies
Surface natural waters are often used as sources of public water supply. Effluent from WWTPs containing high levels of PFASs discharged upstream from a drinking water intake can pose a threat to downstream consumers.
Effective removal of PFASs in drinking water requires the same costly technologies used to remove it from wastewater, with the same strategy of limiting discharges to WWTPs through inlet control.
An additional protection measure for public drinking water supplies can also be implemented by limiting PFASs in upstream discharges.
Along the same lines, a similar mechanism can be employed through a wellhead protection program, to provide better protection of public groundwater supplies.
Current treatment options for PFAS-contaminated water
Treating PFAS-contaminated water before discharge into receiving sources will reduce its build-up in water systems. Current industrial PFAS removal methods for contaminated water are based on physical adsorption technologies, such as granular activated carbon (GAC) and ion exchange (IX) resins; as well as filtration with high-pressure semi-permeable membranes, such as nanofiltration (NF) and reverse osmosis (RO).
Although advanced oxidation techniques are under investigation, these are not yet commercially viable and could be very energy-intensive and expensive. Selecting an appropriate treatment method requires careful consideration of the specific water chemistry, the removal of contaminants and the required quality for treated water.
The composition of wastewater when treated industrially is more complex than that of drinking water and includes other contaminants in addition to PFASs. The properties of these contaminants will affect the selection of the method to be used, the size of the treatment system and operating costs. For example, landfill leachate has organic, inorganic and volatile contaminants, in addition to PFASs, which require disposal.
Each of these treatment technologies has its advantages and disadvantages, as follows:
Granular activated carbon (GAC)
Advantages
- Reduces PFAS levels to ng/L in drinking water.
- Effective for the removal of long chain PFASs.
Disadvantages
- Short-chain PFAS leaks, in particular and frequent replacement of GAC filtering loads.
- Not profitable for water containing other organic compounds, as the GAC is not selective and will become partially saturated with them.
- Does not remove inorganic compounds.
- GAC is a very expensive consumable due to the material cost, labor for loading and unloading and the energy cost of thermal regeneration.
Ion exchange resins
Advantages
- Effective for the removal of anionic and long chain PFASs at the ng/L level.
- Greater adsorption capacity and significantly faster reaction kinetics compared to GAC.
Disadvantages
- Not effective for wastewater containing high TDS levels and/or natural organic matter.
- Less affinity for short chain PFASs.
- Incineration or regeneration of the ion exchange resin is required.
Nanofiltration and reverse osmosis
Advantages
- Effective for both short- and long chain PFASs.
- Capable of treating all types of water contaminated with PFASs.
- High load flow.
- Can be associated with a disposal pond (common in North America) to permanently remove PFAS brine.
Disadvantages
- Possible fouling of the membrane when treating inorganic compounds.
- Concentrated brine management, which can be achieved through high recovery performance to minimize the volume of the separated brine, ensuring there is no precipitation or fouling.
A PFAS removal process can require a number of different technologies; e.g. an upstream reverse osmosis process with a high loading rate followed by a downstream polishing step of GAC or IX resin to meet stringent water quality requirements.
Other technologies for treatment of wastewater containing PFASs
The physical separation technologies (GAC, IX resin, NF and RO) do not destroy the PFASs, but only separate them from contaminated water on adsorbent materials or in concentrated brine. Disposal of PFAS-contaminated absorbents or concentrated PFAS brine can lead to secondary contamination.
Technologies for permanent PFAS degradation are based on high-energy incineration or advanced oxidations, including electrochemical oxidation, microwave heat treatment, photolytic degradation, pyrolysis and sonochemistry.
These extreme PFAS degradation pathways are very expensive, especially when the volume and flow of PFAS wastewater is large.
The best solution is to use other relatively cost-effective technologies to reduce the volume of wastewater with PFASs first, then concentrate the PFAS to its highest allowable concentration before the removal of contaminants.
Wastewater with a high concentration of PFASs can be transferred to a well for underground storage, or undergo final destruction by specialized PFAS degradation.
New advances in desalination technologies (ultra-high pressure reverse osmosis, minimum liquid discharge (MLD) and zero liquid discharge (ZLD) with an evaporation-crystallization system available from Condorchem Envitech.
Extreme Reverse Osmosis can help reduce the volume of wastewater containing PFASs and concentrate them to a level that was previously impossible.
Vacuum evaporation: A PFAS treatment solution
An industrial coating company based in Michigan, USA had a problem with PFASs in its wastewater and untreated groundwater process. This factory has used diamond chrome technology (DCP) since the 1950s.
As part of its process, surfactants with PFASs formed a floating layer in the chrome vats and were used to suppress gaseous emissions of hexavalent chromium, volatile organic compounds and other contaminants. These were then taken to the rinse baths and drainage and storm sewers before leaking into underground aquifers.
The effluent flow rate was 6,000 gallons per day, and was treated to achieve zero discharge; while obtaining condensate of sufficient quality to be reused in the industrial process.
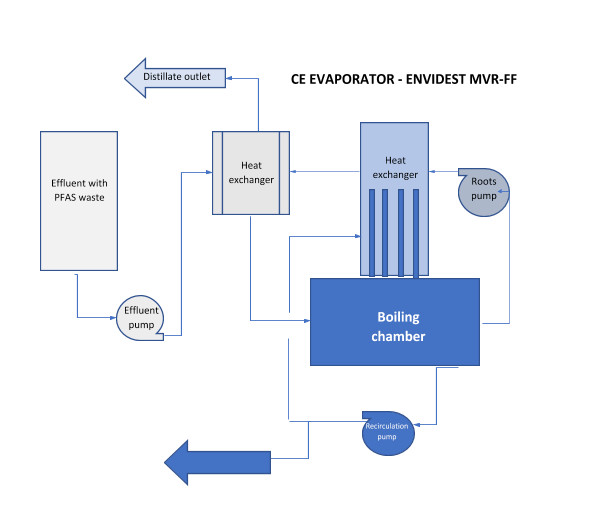
Condorchem Envitech recommended a process based on vacuum evaporation, using an Envidest MVR FF evaporator with falling film and forced circulation via mechanical vapor compression.
This technology optimizes heat exchange, leading to significant energy savings. The process also enables automatic discharge and the evaporator vacuum system with automatic cleaning within the evaporator itself.
Fig. 1 – Block diagram of effluent containing PFAS treated by evaporation
Evaporation systems can be integrated into a complete solution to remove these contaminants and concentrates, while recovering clean water for reuse and ensuring that companies comply with these strict environmental regulations.
Condorchem Envitech is an environmental engineering company with over 25 years of experience in the water industry, specializing in concentration technologies to treat the most difficult wastewater streams.
One of the main benefits of Condorchem equipment is that each application is different, and so it can be completely flexible with its study and design. The idea is to provide a complete solution for each effluent problem.
Condorchem designs take into account issues such as internal space for the application, days of operation and the flow and variety of discharge to be treated.
CE has over 400 projects worldwide, with more than 200 achieving zero liquid discharge. The objective is to provide the best technical solution at the best price, with the best quality equipment, at all times.
Summary
PFASs have been used since the 1950s. PFOS production started in 1948, and was used in large quantities until 2000, both to provide inert liquids of low surface tension, and for solid surfaces with specific properties.
These substances are highly resistant to degradation and therefore useful in processes with high temperatures or in contact with strong acids or bases. However, because of this resistance, they tend to accumulate over time and are highly dangerous for both humans and the environment.
Animal studies carried out showed them to be global, persistent and cumulative pollutants, with levels which may be of concern in the near future. This has caused significant public alarm and alerted the different regulatory agencies.
To the traditional, financially viable solutions to separate PFASs – reverse osmosis (RO) membranes, adsorption on granular activated carbon (GAC) and separation with ion exchange resins (IX) – have been added others, such as vacuum evaporation, which make it possible to farther concentrate the waste of these pollutants at competitive implementation and operation costs.
References and information on the Internet
https://espanol.epa.gov/espanol/informacion-basica-sobre-pfas
http://www.newmoa.org/events/docs/241_213/CrimiPFASWebinarDec2106.pdf
https://www.tekcrispy.com/2018/10/10/solucion-tratar-aguas-pfas/
https://es.wikipedia.org/wiki/Sustancias_perfluoroalquiladas