Sections
- Basic characteristics of lithium
- Applications of lithium
- Recycling of electronic devices using lithium ion batteries
- Extraction and refining of lithium
- Recycling batteries and recovery of lithium
- Conclusions
Basic characteristics of lithium
Lithium (Li) is the lightest metal, with a density half that of water. Like the other alkali metals, it is monovalent and highly reactive, although less so than sodium, which is why it is not found in its free form in nature.
It is a moderately abundant element; present in the Earth’s crust at 65 ppm, ranking below Ni, Cu, and W, and above Cr and Sn.
Alongside H and He, Li is one of the few elements formed during the Big Bang. All others were synthesized through nuclear fusion in stars on the main sequence or during supernova explosions.
Its basic physico–chemical properties are:
- Density: 535 kg/m³
- Mohs hardness: 0.6
- Appearance: Silvery-white, gray solid
- Atomic mass: 6.941
- Atomic radius: 167 pm
- Oxidation state: Strong base
- Crystal structure: Body-centered cubic
- Melting point: 453.69 K
- Boiling point: 1615 K
- Specific heat: 3582 J/(kg·K)
In terms of properties, lithium is a soft, silvery-white metal with a very low Mohs hardness of 0.6. Its atomic mass is ~6.94 amu, and it has an atomic radius of 167 pm. Lithium’s crystal structure is body-centered cubic. It has a melting point of 453.7 K and a boiling point of 1615 K.
Notably, lithium has an extremely high specific heat capacity (3582 J/(kg·K)), the highest of any solid element. It also exhibits a high electrochemical potential (the most negative standard reduction potential among metals). These traits underlie lithium’s value in energy storage and heat transfer applications.
Applications of lithium
Lithium is often referred to as “white oil” or “white gold” due to the central role it currently plays, and will continue to play, in the global energy landscape.
Lithium-ion batteries are by far the largest consumer of lithium globally, outpacing all other uses combined.
Its properties make lithium ions the ideal ingredient for battery manufacturing. Thanks to its high specific heat, lithium is used in heat transfer applications, and due to its high electrochemical potential, it serves as a suitable anode material for batteries in electric vehicles, smartphones, and various electronic devices. Li-ion batteries are highly energy-dense, enabling longer runtimes and driving ranges in devices and EVs.

Lithium also has several other uses, although their consumption is relatively small compared to its application in batteries:
- Pharmaceuticals: Lithium salts, particularly lithium carbonate (Li₂CO₃) and lithium citrate, are used in the treatment of mania and bipolar disorder, and more recently, in unipolar depression. They act as mood stabilizers, believed to be linked to their effects on serotonin function. Lithium is present dissolved in blood plasma and/or red blood cells.
- Drying agents: Lithium chloride and lithium bromide are highly hygroscopic, making them excellent desiccants. Lithium bromide, in particular, is used in absorption heat pumps, along with other compounds such as lithium nitrate.
- Lubricants: Lithium stearate is widely used as a high-temperature lubricant.
- Chemical synthesis: Lithium serves as a reagent in the synthesis of organic compounds.
- Air purification: Lithium hydroxide is used in spacecraft and submarines to scrub carbon dioxide from the air.
- Alloys and materials: Lithium is a common component of aluminum, cadmium, copper, and manganese alloys used in aerospace construction. It has also been successfully applied in the manufacture of ceramics and optical lenses, such as the 5.08 m diameter telescope at Mount Palomar.
- Nuclear applications: Lithium has two stable isotopes, Li-6 and Li-7, with the latter being the most abundant (92.5%).
Recycling of electronic devices using lithium ion batteries
The processing and recycling of waste from electrical and electronic equipment, such as computers, car batteries, e-bike and scooter batteries, tablets, headphones, or mobile phones, among others, is more important than ever due to the rapid increase in the consumption of these products.
Current industrial recycling efforts mostly target the more valuable metals in the battery (such as cobalt and nickel), while lithium itself is often overlooked or discarded. This may seem counterintuitive, but historically it has been an economic reality: lithium’s market price has been low relative to cobalt or nickel, so there has been less financial incentive to recover it. Moreover, lithium recovery processes can be technically challenging and costly, which has made lithium recycling expensive and often not profitable under past market conditions.
Today, when lithium-ion batteries are sent to recycling, typically the high-value metals like cobalt, nickel, and copper are extracted first. For example, cobalt from Li-ion batteries can be recovered as lithium cobalt oxide or other compounds, due to cobalt’s high market price and critical supply status. Lithium, by contrast, has often ended up in the slag or waste stream in smelting-based recycling processes. This represents a lost opportunity, as lithium is a finite resource and demand is soaring.
Recovering lithium from end-of-life batteries can significantly reduce the need for mining new lithium and bolster the supply of this critical material. It also mitigates the environmental impact by reusing what has already been extracted.
Nevertheless, the rapid rise of electric mobility and the global transition toward cleaner energy are accelerating innovation in battery recycling technologies. Governments are also enacting regulations and setting targets that encourage recycling and resource circularity. In the next sections, we will explore the global landscape of lithium battery recycling and the evolving technologies aiming to make lithium recovery viable.
Global recycling landscape
Recent research presents conflicting figures.
One 2023 study estimated that around 59% of end-of-life lithium-ion batteries are recycled globally. However, projections based on supply-demand modeling suggest that less than 20% of lithium supply by 2050 will come from recycling. This disparity highlights ongoing challenges in accurately measuring and scaling lithium recovery worldwide.
Regionally, there are big differences:
- As of 2023, worldwide lithium battery recycling capacity was estimated at over 300 GWh per year
- China has become the global leader in LIB recycling capacity, with more than 80% of global capacity located in China.
- Europe and the United States each account for under 2% of global recycling capacity, although both regions are now investing heavily to catch up.
- Ambitious targets in the European Union aim to dramatically increase battery collection and materials recovery, with some forecasts suggesting that by mid-century, recycled lithium could supply up to 50% of Europe’s lithium needs.
The global lithium-ion battery recycling market is expanding rapidly alongside the boom in battery usage. In 2024, the LIB recycling market was valued around USD 10.3 billion, and is projected to reach USD 98.4 billion by 2034, growing at an astonishing CAGR of ~25.4% over the decade.
For perspective, the overall battery recycling market (including all battery types) is expected to grow from about USD 22.8 billion in 2024 to USD 41.7 billion by 2030. These numbers reflect the sheer volume of batteries that will be retiring and the increasing value of the materials inside them. The push for resource availability, especially for critical metals like lithium, nickel, and cobalt, means that recycling is becoming a strategic industry in its own right.
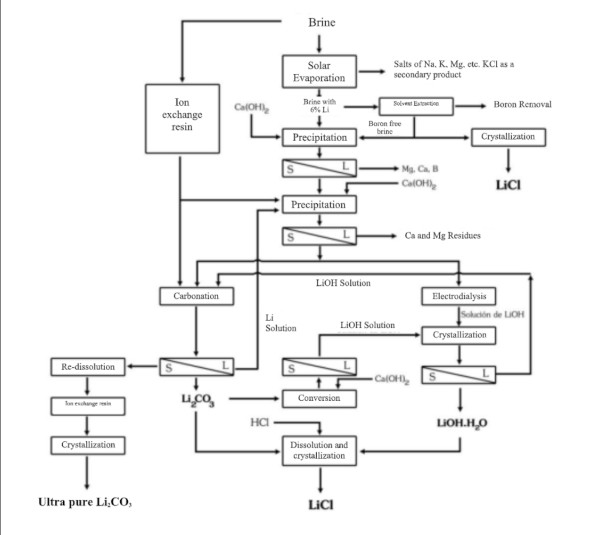
Extraction and refining of lithium
hard rock mining
Australia, the world’s largest lithium producer, mines lithium from mineral ores such as spodumene (LiAlSi₂O₆). Spodumene mining involves traditional excavation of pegmatite ore, followed by crushing, heating, and chemical treatment to produce lithium concentrates.

While effective, this process is energy-intensive, costly, and environmentally impactful. Hard rock extraction and processing typically have higher operating costs (estimated around $4,200–4,500 USD per ton of lithium carbonate equivalent) compared to brine extraction.
Brine extraction
In South American countries like Chile, Bolivia and Argentina, as well as in China, lithium is often extracted from vast salt-flat brines.
A large share of global lithium production comes from these brines, whose production cost is significantly lower than that of mineral deposits (according to John McNulty: 1,500–2,300 USD/ton vs. 4,200–4,500 USD/ton, respectively).

Lithium brine extraction is carried out through pumping, and its concentration can be achieved either by adsorption using a selective adsorbent or by evaporation in shallow ponds built for this purpose.
1. Evaporation not only increases the concentration of salts but also causes certain salts to precipitate once they reach saturation. Over 12–18 months of evaporation, the lithium concentration in brine increases from a very dilute level (~0.1% Li) to a few percent, precipitating out salts sequentially (NaCl, KCl, etc.) along the way. The final concentrate is processed to yield lithium carbonate or lithium chloride.
The advantages of natural evaporation are:
- It consumes no energy
- Clearly cheaper than mining
- It requires few chemical reagents
Its disadvantages include:
- The necessity of using an additional separation method
- The accumulation of waste
- The consumption of enormous quantities of water in inherently dry regions
- Strong dependence on local weather conditions (evaporation rate and rainfall).
2. Adsorption offers the advantages of being unaffected by the composition of the brine (it can even be applied to low-lithium brines, as has been experimentally demonstrated with seawater), as well as being independent of local weather conditions. In addition, it generates limited waste. However, its drawbacks include the need for chemical reagents, the high cost and complexity of the adsorption equipment, and the elevated cost of the adsorbent itself.
The largest global lithium production from brines comes from the Salar de Atacama in Chile, where the evaporation method is applied. Extensive operational data are available for this process, making it possible to compare with the Salar de Uyuni in Bolivia.
- The Atacama brines are richer in lithium (as well as potassium and boron) compared to those of Uyuni. Consequently, the magnesium-to-lithium (Mg/Li) ratio, which is detrimental to lithium concentration, is 6:1 in Atacama and 19:1 in Uyuni.
- Bolivia’s lithium reserves and resources are found in brines, which have an approximate density of 1,200 grams per liter (g/L). Thus, a lithium concentration of 0.1% by weight corresponds to 1,000 parts per million (ppm) or 1.2 g/L.
- While evaporation and annual precipitation rates in Atacama are 3,200 mm/year and 10–15 mm/year, respectively, in Uyuni they are 1,500 mm/year and 200–500 mm/year. In other words, Uyuni has lower evaporation and significantly higher rainfall, which considerably slows down the evaporation process.
- In Atacama, the evaporation process that concentrates lithium from 0.15% to 6% (a 40-fold increase) takes 12 to 18 months; it is therefore expected that in Uyuni this process will take much longer.
A laboratory study titled “Chemical Treatment of Brines from the Salar de Uyuni-Potosí”, conducted in France in 1987 under the UMSA-ORSTOM Agreement (French Institute of Scientific Research for Development), simulated the conditions of evaporation ponds in five containers. The study determined that sodium chloride (NaCl) precipitates first, followed shortly thereafter by potassium chloride (KCl).
Since magnesium chloride (MgCl₂) cannot be separated by evaporation, it must be precipitated as magnesium hydroxide (Mg(OH)₂) by adding lime.
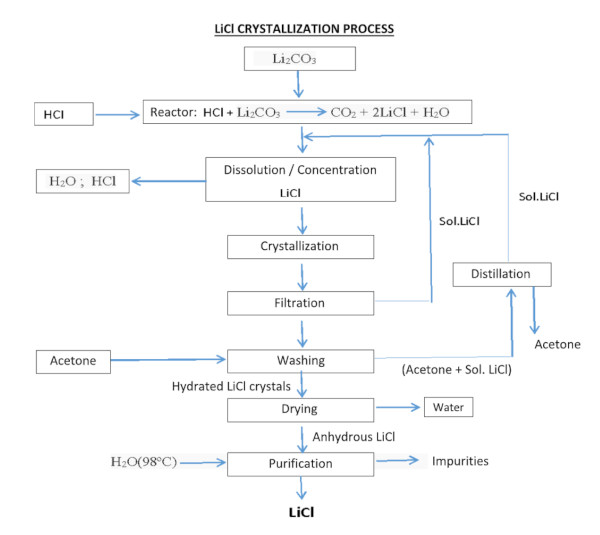
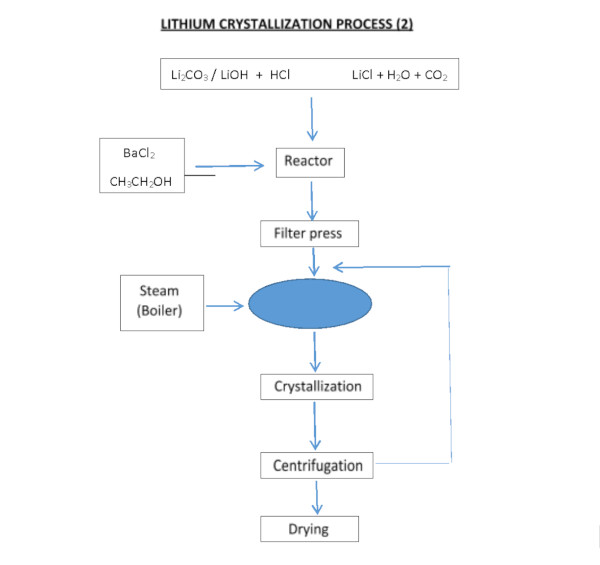
The process, designed to obtain lithium chloride from carbonate or lithium hydroxide, can be achieved by reacting with hydrochloric acid:
Li2CO3 + 2HCl === 2LiCl + H20 + C02
LiOH·H2O + HCl === LiCl + 2H2O
Importance of precipitation and refining of lithium carbonate
The lithium chloride (LiCl) obtained by any method must be purified, dried, and crystallized. Despite the high lithium content of the Salar de Atacama and the extensive experience in its extraction, recovery rates have been reported at only 42%.
For use in the manufacture of batteries for electric vehicles, lithium chloride must reach a minimum purity of 99.95%. Therefore, the LiCl obtained by precipitation requires further refinement through multiple reactions and recrystallization stages, and in some cases, by means of ion-exchange resins.
Since the refining process is costly and recovery decreases after each stage (estimated at approximately 70% during refining), the higher the required purity of LiCl, the more disproportionately its price increases.
In summary, primary lithium extractionrequires significant land use, water consumption, and energy input, and often results in substantial waste or environmental disruption.
Recovering lithium through recycling can dramatically reduce these impacts. Indeed, recycling lithium-ion batteries avoids new land disruption and can cut greenhouse gas emissions by 17–61% compared to producing lithium chemicals from virgin mining. It also typically uses far less water than extracting lithium from brine sources.
From both an environmental and economic perspective, there is a strong incentive to recycle lithium from batteries as a supplement or alternative to mining, especially as global demand continues to climb.
Precipitation and Refining of Lithium Carbonate process
Whether starting from brine or hard rock, one common end product of lithium extraction is lithium carbonate (Li₂CO₃), which is widely used in battery cathode production (and other applications). Converting lithium chloride or other intermediates into lithium carbonate involves precipitation reactions. For example, adding sodium carbonate (soda ash) to a lithium chloride solution will precipitate lithium carbonate:
2 LiCl (aq) + Na₂CO₃ (aq) → 2 NaCl (aq) + Li₂CO₃ (s)↓
The crude lithium carbonate is then filtered, washed, and refined to battery grade if needed. Alternatively, lithium chloride can be converted to lithium hydroxide (LiOH) by reaction with lime (calcium hydroxide), as is often done when LiOH is desired for certain battery chemistries.

A key point is that achieving the ultra-high purity levels for battery-grade lithium carbonate/hydroxide is challenging. Even the world’s richest brine (Atacama, Chile) achieves only ~42% recovery of lithium in the initial evaporation step, and further refining yields losses. As mentioned, each purification stage (re-crystallization, ion exchange, etc.) can reduce overall yield to around 70% of the input. Thus, by the time one gets a final 99.9% Li₂CO₃ product, a large fraction of the original lithium may have been left behind in mother liquors or waste streams. These inefficiencies in primary extraction highlight why closing the loop with recycling is attractive – once lithium is in a battery, keeping it cycling through reuse and recycling can be far more sustainable than continually mining fresh material.
It’s worth noting that lithium carbonate equivalent (LCE) is the standard unit for lithium industry metrics; 1 gram of lithium is equivalent to 5.32 grams of Li₂CO₃. Battery producers are increasingly demanding lithium in the form of high-purity lithium hydroxide (for high-nickel cathodes) or lithium carbonate (for LFP and other cathodes), putting pressure on the supply chain to deliver very pure chemicals either from mines or recycling facilities.
Recycling batteries and recovery of lithium
Aware of the need to improve lithium recovery from spent batteries – especially given concerns of future scarcity and price increases – researchers and companies worldwide have been developing and piloting various lithium battery recycling processes. Broadly, recycling processes involve a combination of physical and chemical steps to isolate and reclaim valuable components. A typical recycling pipeline goes through the following general phases:
- Collection – Used batteries are collected from consumers, manufacturers (scrap), or other sources.
- Preprocessing & Discharge – Batteries are sorted by type/chemistry and fully discharged to eliminate any residual charge (for safety).
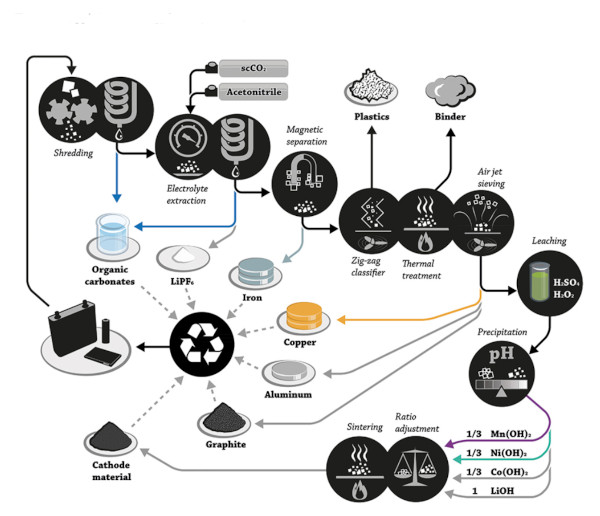
- Dismantling/Shredding – Packs/modules are dismantled. Cells may be shredded or crushed in a controlled manner. This yields mixtures of materials.
- Physical Separation – Mechanical and physical techniques (screening, magnetic separation, density separation, etc.) separate out components: steel casings, aluminum and copper foils, plastics, etc. The goal is to concentrate the black mass – a powder containing cathode and anode active materials – by removing other materials.
- Thermal/chemical Decontamination – Processes like low-temperature thermal treatment or solvents may be used to remove electrolytes, binders, and other organics, which reduces hazards and prepares the black mass for metallurgical recovery.
- Metallurgical Recovery – Finally, the critical metals (Li, Co, Ni, Mn, etc.) in the black mass are extracted and purified via appropriate chemical processes. This can involve high-temperature smelting (pyrometallurgy), chemical leaching (hydrometallurgy), or other novel techniques discussed below. The output might be elemental metals, or more commonly, chemical compounds (oxides, sulfates, carbonates, etc.) ready for reuse in new battery production.
Each of these steps can be executed in different ways depending on the specific process and the chemistry of the batteries being recycled. There is no one-size-fits-all: recycling a laptop battery with lithium cobalt oxide (LCO) cathode can differ from recycling an EV battery with nickel-manganese-cobalt (NMC) cathodes or an LFP (lithium iron phosphate) battery, for example. Effective recycling often requires tailoring the process to the battery chemistry and form factor.
Physical – Chemical Process (Pyrometallurgy vs. Hydrometallurgy)
Pyrometallurgical process
Historically, the pyrometallurgical approach was the most common route for recycling lithium-ion batteries.
In a pyrometallurgical process, batteries (often after some pretreatment like drying or dismantling) are smelted at high temperatures (800–1400 °C) in a furnace. The organic materials and plastics are burned off, and the metals are melted. Valuable transition metals like cobalt, nickel, and copper are collected in an alloy (metal melt), while lithium (along with aluminum, manganese, and other light elements) reports to the slag (an oxide melt).
The slag from such smelters is sometimes used in the cement/construction industry or can be further processed to recover lithium, but doing so is often not cost-effective. In essence, traditional pyrometallurgy does not directly recover lithium, which ends up in a slag that might be stockpiled for future treatment or discarded.
The advantages of pyrometallurgical recycling are:
- Relative simplicity and robustness.
- It can process assorted battery scrap without extensive sorting or preprocessing.
- It yields alloy products from which metals like nickel and cobalt can be later refined.
However, the disadvantages are significant:
- The process consumes a lot of energy (maintaining >1000 °C).
- Requires off-gas treatment for toxic fumes.
- It has low overall recycling efficiency for lithium and other light elements.
Lithium recovery from pyrometallurgical slag is possible but requires additional chemical treatment with high energy or reagents, making it economically unattractive in many cases. In short, pyrometallurgy tends to prioritize cobalt, nickel, and copper recovery; it is an established but inflexible approach with limited ability to reclaim lithium or graphite in a useful form.
Hydrometallurgical process
In recent years, focus has shifted toward hydrometallurgical processes for battery recycling.
A hydrometallurgical process typically involves taking the shredded or pre-separated battery materials and leaching them in chemical solutions to dissolve the metal ions. Acids (like sulfuric, nitric, or organic acids) often serve as leaching agents, sometimes with reducing agents to help dissolve metallic forms.
For example, black mass can be leached with sulfuric acid in the presence of hydrogen peroxide as a reducing agent, bringing Li⁺, Co²⁺, Ni²⁺, etc. into solution. The leachate is then subjected to separation and purification steps, such as solvent extraction, selective precipitation, or ion exchange, to recover each metal in a saleable form.
Hydrometallurgy offers the following advantatges:
- It operates at much lower temperatures (often below 100 °C).
- It can be more selective in what it recovers.
- It also can achieve higher overall yields. For instance, modern hydrometallurgical flowsheets can recover nearly 100% of the lithium and cobalt, around 98% of manganese, and high percentages of nickel from batteries.
- The output can be high-purity compounds like lithium carbonate, cobalt sulfate, nickel sulfate, etc., which are directly reusable in battery manufacturing.
The trade-off is:
- Hydromet processes often involve multiple stages and the use of chemical reagents, generating liquid waste that must be treated.
- They can be more complex to operate and sensitive to feed composition, requiring good front-end sorting and preprocessing.
Despite these challenges, hydrometallurgy is generally viewed as a more sustainable and efficient approach than pyrometallurgy for LIB recycling, given its potential for much higher recovery rates and lower energy use. Indeed, many new recycling startups and research projects are pursuing hydrometallurgical or hybrid hydro/pyro techniques to maximize material recovery.
In practice, some combined approaches are used. For example, a low-temperature thermal treatment (to remove organics and make materials brittle) followed by hydrometallurgical leaching.
There are also biometallurgical methods (bioleaching using microbes to generate leaching acids) under development, which promise lower chemical usage, though they can be slower.
Example hydrometallurgical process
One illustrative process reported for lithium battery recycling involves ammonia-based leaching to remove interfering metals, followed by acid leaching for the active materials.
- Manual disassembly of the battery and crushing of cells to a fine powder (~500–800 µm)
- The powder is first leached with ammonium hydroxide to selectively dissolve and remove aluminum and copper (from current collectors). Removing these base metals first prevents them from consuming reagents or contaminating later steps.
- The remaining solid (containing cathode and anode materials) is then leached with a strong acid (such as sulfuric acid). This brings lithium, cobalt, manganese, and nickel into solution.
- Reported recovery efficiencies in the leachate are extremely high: on the order of 96.0–99.9% for each of Li, Co, Mn, Ni.
- Subsequent steps neutralize the solution and precipitate individual elements: e.g. adding sodium hydroxide and sodium bicarbonate causes precipitation of manganese as MnCO₃, cobalt as Co(OH)₂, and lithium as LiHCO₃ (which can be calcined to Li₂CO₃).
- Final evaporation and crystallization yield purified compounds, with overall metal recoveries above 96%. This kind of process demonstrates that near-quantitative lithium recovery is technically achievable.
The challenge lies in doing it economically at scale – optimizing reagent use, handling a variety of battery types, and managing the waste streams.
Note: Lithium carbonate (Li₂CO₃) remains the most widely traded lithium compound in the industry (with lithium hydroxide growing in use). It’s useful to remember conversion: 1 gram of lithium metal ≈ 5.32 grams of Li₂CO₃.
Mechanochemical Recycling (Innovation)
In response to the limitations of conventional hydro and pyrometallurgy, researchers have been exploring mechanochemical recycling methods.
Mechanochemistry involves using mechanical energy (such as high-energy milling/grinding) to induce chemical reactions in solids without requiring large amounts of heat or solvents.
A groundbreaking 2023 study by Dolotko et al. introduced a mechanochemically-induced lithium extraction process that is acid-free and energy-efficient.
In this process, the cathode material from spent LIBs is milled together with a reducing agent (in this case, elemental aluminum powder, which is actually the material from the battery’s foil current collector). Through intense milling, a reaction occurs that reduces lithium metal oxides (like LiCoO₂, LiFePO₄, NMC, etc.) and yields water-soluble lithium compounds and metallic alloy byproducts. Two process variants were developed:
- After milling, one route directly leaches the powder in water.
- Another adds a carbonization step to convert lithium compounds into lithium carbonate before leaching.
The key results were very promising:
- The mechanochemical approach achieved lithium recovery rates up to 70–76% without using any acids or high-temperature furnaces.
- Crucially, this method proved effective on all major cathode chemistries (LCO, LMO, NMC, LFP) including mixtures of different cathodes, addressing a big challenge since real-world battery scrap is often a mix of chemistries.
- The end product was battery-grade Li₂CO₃ with purity around 99.9% after minimal purification.
- Mechanochemical recycling could thus bypass the need for strong chemical leachants and could simplify the process flow.
- Its energy consumption is mainly in the milling step, but overall it’s touted as more energy-efficient than pyrometallurgy.
While still at research scale, this approach highlights a trend toward greener, “solvent-less” recycling techniques. If scaled up, mechanochemical processes might allow recycling plants to recover lithium and other metals with lower emissions and without generating large volumes of toxic waste. It’s an exciting development that complements other hydrometallurgical innovations.
Direct Recycling and Cathode Reuse
Another important emerging approach is direct recycling (also called direct cathode regeneration).
Unlike metallurgical processes that break cathodes down into elemental constituents (metals or simple salts) and then require re-synthesis of cathode materials, direct recycling aims to preserve the cathode’s structure and composition for reuse. In other words, rather than converting everything to, say, lithium carbonate and cobalt sulfate, why not directly recover LiCoO₂ or Li(NiMnCo)O₂ in a form that can be re-used in a new battery? If successful, this could skip many energy-intensive steps.
In a direct recycling process, the spent cathode material is typically collected (after physical separation of electrode sheets), and then treated to restore its performance. This might involve heating, chemical relithiation, and/or removal of impurities that accumulated during battery use. The goal is to make the recovered cathode powder as good as new. For example, if an NMC cathode has degraded (losing some lithium into the anode or developing defects), direct regeneration might add lithium back (e.g. soaking cathode in a lithium salt solution and heating) and heal structural defects. Studies have shown it’s possible to restore lithium-ion cathodes to near-original capacity without completely breaking them down. This has huge appeal because it reduces energy consumption and chemical use dramatically – you don’t have to separate every element and then make the cathode from scratch.
According to Gaines et al. (2021) and others, direct cathode recovery can be more economical and environmentally friendly for certain chemistries. There are two aspects to direct recycling: regeneration (bringing a cathode back to original specs) and upcycling (improving it to have even better performance or using it in a different application). For instance, a recovered cathode could potentially be modified (doped or have coatings applied) to perform better than the original.
Various methods for direct recycling are under development, including:
- Hydrothermal relithiation.
- Sol-gel methods.
- Solid-state lithiation.
Each has trade-offs in terms of energy use, completeness of restoration, and scalability. Some approaches require high-temperature annealing (which uses energy but can process large batches), whereas others use solutions at lower temperatures but might need more steps.
The benefits of direct recycling are clear:
- By avoiding the “back to black mass” route, we save the considerable energy and reagents otherwise spent to isolate metals and then manufacture new cathode material.
- It’s been noted that current recycling sends everything to a generic intermediate (like black mass or basic salts), which is a waste of the effort already invested in making the complex cathode materials. Direct recycling attempts to “skip the middleman” of refining to basic compounds, instead directly refurbishing the cathode or anode material. This can potentially be cheaper and produce cathode materials ready for reuse in new cells with minimal additional processing.
Several initiatives, such as the ReCell Center in the U.S., are actively researching direct cathode recycling for LIBs. Challenges include:
- Ensuring the recovered cathodes meet strict quality and consistency requirements for new batteries
- The process works for next-generation chemistries.
Still, direct recycling is a promising strategy that could complement traditional metallurgical recycling – reclaiming high-value cathodes when possible, and using hydro/pyro methods for the remainder of materials.
Our crystallizers for the refining or recycled lithium
We supply end-to-end vacuum crystallization systems for Li-ion recycling plants.
Crystallizers are essential to turn lithium solutions into battery-grade Li₂CO₃/LiOH. Evapo-crystallization is ideal to obtain the desired purity, yield, and particle size.
Our MVR crystallizers and multiple-effect evaporators are designed to maximize efficiency, reliability and durability. Besides lithium purification, they deliver excelent results for water reuse and recovery of byo-products such as Na₂SO₄.
In modern lithium recycling plants, the most efficient and reliable process goes as it follows:
- First, hydrometallurgy process and polishing brin lithium into solution, while dissolving and separating metals.
- Next, vacuum crystallization is the best technology to make saleable salts and recover water and by-products. They achieve the desired purity, yield, and PSD, producing battery-grade Li₂CO₃/LiOH.
This is where crystallizers really shine:
- Only a controlled crystallization + re-crystallization system reliably delivers ≥99.5–99.9% Li₂CO₃/LiOH with tight PSD. Precipitation alone rarely hits battery specs consistently.
- Impurity rejection: Counter-current cold washing and mother-liquor management push Na/K/Mg/Ca/B down to ppm levels.
- Vacuum MVR crystallizers are a proven, cost-effective technology. Tthey use electricity, integrate with renewables, enable water reuse and recover by-products (ZLD).
- Sodium sulfate and other valuable resources can be crystallized and sold as by-products instead of having to be managed as liquid waste.
Emerging Techniques
In addition to the major categories above, there are other novel processes and improvements being explored:
“Design for Recycling” and Automated Dismantling
One way to improve recycling is to start at the battery design stage. Manufacturers are beginning to consider end-of-life disassembly in their designs (using fasteners that are easier to undo, modules that can be opened, etc.). The concept of designing batteries for easier recycling could greatly reduce the labor and cost of the initial disassembly phase. Additionally, smart identification and sorting of batteries using technologies like RFID tags or labels can help recyclers efficiently separate batteries by chemistry and condition. This is important because recycling processes are most efficient when the feed is uniform and known.
High-Efficiency Electrochemical Recovery
The original article described an innovative electrochemical process developed by researchers in Argentina (Ernesto Calvo et al.). In this process, lithium-containing brine is pumped through a cell with special electrodes that selectively capture lithium ions on one side and chloride on the other. By switching the electric potential, lithium is released from the electrode into a fresh solution as high-purity lithium chloride. This technique basically acts like a “lithium sponge” battery that can extract lithium without evaporating huge amounts of water. It was notable for producing no polluting waste, as the remaining brine (depleted of lithium) is returned to the salar, and solar energy can power the system. Such direct lithium extraction (DLE) methods are very promising for both geothermal brines and possibly for recycling wash solutions, since they target lithium ions very specifically.
Membrane and MOF-based Lithium Separation
A breakthrough in materials science has introduced metal-organic framework (MOF) membranes that can filter lithium from complex solutions.
Researchers from Monash University, CSIRO, and UT Austin developed a MOF membrane inspired by biological ion channels, achieving highly selective lithium ion passage while rejecting other ions.

This approach was demonstrated for lithium extraction from seawater, which contains a tiny concentration of lithium (~0.17 ppm). Traditional reverse osmosis can desalinate water but doesn’t specifically pick out lithium. The MOF membrane, however, can dehydrate lithium ions as they pass through, effectively separating lithium from sodium, magnesium, and other ions.
The process simultaneously produces fresh water. What’s remarkable is that these MOF filters can operate at much lower pressure than conventional RO membranes (since they don’t rely purely on size exclusion). This translates to significantly lower energy consumption. If scaled up, such membranes could allow lithium to be harvested from abundant sources like seawater or brine in an energy-efficient manner, potentially yielding lithium chloride solutions that could then be crystallized to lithium hydroxide or carbonate.
While this is more relevant to primary lithium production than battery recycling, it exemplifies the innovative technologies emerging in the broader lithium recovery space. In a recycling context, advanced membranes might be used to refine lithium from leach solutions or to treat recycling effluents, making the overall process more sustainable.
Selective Lithium Precipitation (LiOH)
Some companies have explored chemical methods to speed up lithium recovery from brines without large pond evaporation. One such process (patented by Posco and piloted in Argentina) involves adding phosphate to precipitate lithium as insoluble lithium phosphate (Li₃PO₄), then reacting it with lime to produce lithium hydroxide (LiOH) directly. The chemistry is:
LiCl (aq) + H₃PO₄ → Li₃PO₄ (s) ↓ + HCl
,
LiCl (aq) + H₃PO₄ → Li₃PO₄ (s) ↓ + HCl,
then:
{Li₃PO₄ + Ca(OH)₂ → 3 LiOH + Ca₅(PO₄)_3OH (hydroxyapatite)↓}.
Essentially, lithium is pulled out of solution quickly as Li₃PO₄, then converted to LiOH. This process avoids waiting on solar evaporation and can yield high-purity LiOH in a matter of days instead of months. The trade-off is managing the by-products: calcium phosphate (hydroxyapatite) and gypsum (CaSO₄·2H₂O if sulfuric acid is used to regenerate phosphoric acid).
Posco’s method reportedly cuts the lithium recovery time dramatically, though it may leave behind some waste sludge. It’s a reminder that alternative chemistries can sometimes leapfrog conventional processes, and similar principles might be applied to fast-track lithium recovery in recycling as well (for instance, precipitating lithium from leachate as Li₃PO₄ or Li₂CO₃ in one step).
Adsorption Methods
Lithium-selective adsorbents have been researched for decades, originally for extracting lithium from brines and seawater. Compounds like manganese oxide (Lambda-MnO₂), titanium dioxide, or aluminum hydroxide can be structured to selectively take up lithium ions from a solution and then release them in a concentrated form. Some lithium adsorbents achieve capacities of 20–35 mg Li per gram of adsorbent when processing high-lithium brines. While mostly geared toward primary lithium extraction, adsorbents could also be used in recycling – for example, to clean up dilute lithium-containing effluents or to capture lithium from low-grade scrap leachate. The advantage of adsorption is that it can be tuned to target lithium even at low concentrations (like in mine tailing water or very dilute solutions). New nanomaterials and MOFs are being explored to improve capacity and selectivity. This is yet another tool that could find a place in future lithium recovery flowsheets, especially as we aim for zero-waste, closed-loop processes.
Second-Life Applications for Lithium Batteries
Recycling is not the only end-of-life pathway for lithium-ion batteries. An increasingly popular concept is giving batteries a “second life” before they are eventually recycled.
Second-life battery use means taking battery packs that are no longer fit for their original application (e.g. an EV battery that has dropped below the desired range capacity for a car) and reusing them in a different, less demanding application. For instance, an electric vehicle’s battery is typically retired once it loses about 20%–30% of its original capacity (this might occur after, say, 8–10 years in a car). At that point, the battery might only hold ~70–80% charge, which reduces vehicle range. However, that same battery can still be very useful for stationary energy storage where weight and space are less critical and it doesn’t need to deliver high bursts of power.

Examples of second-life uses:
- Retired EV battery packs (or modules) can be repurposed for residential or commercial energy storage systems, storing energy from solar panels or providing backup power.
- They can be aggregated to build grid-scale storage for renewable energy smoothing and peak shaving. For instance, Nissan and other automakers have pilot projects powering street lights or buildings using second-life EV batteries.
- Repurposing battery modules to power electric forklifts, golf carts, or as portable power units.
By employing used batteries in this way, we extract additional value and postpone the recycling stage. Studies show a second-life battery could last another 5-10 years in these applications, depending on usage patterns.
The environmental benefit of second-life is significant:
- It delays the energy and impacts of recycling and new battery manufacturing
- It ensures the full useful life of the battery’s materials is realized.
- It’s effectively an extension of the product lifecycle, which improves overall sustainability metrics (less frequent manufacturing, lower total resource use per kWh delivered over the battery’s life).
However, second-life is not without challenges. One major consideration is the state-of-health assessment and reconfiguration of used batteries. To safely reuse a battery, it must be tested and graded (since not all used batteries will be equal – some may have faults or degraded cells). Then, multiple used modules might need to be reassembled into a new battery system with suitable controls. Standardization can help; for example, EV batteries from the same model car are identical, so it’s easier to repurpose thousands of similar packs than a mix of many types. In contrast, industrial batteries (for machinery, etc.) often come in diverse designs and smaller volumes, making a standardized second-life approach harder. There are also questions about warranty, liability, and how to collect and transport used batteries for second-life use.
Nonetheless, many experts view second-life utilization as complementary to recycling. The ideal lifecycle might be:
Use in EV → second-life in stationary storage → then recycling.
This way, the battery yields the maximum economic value and delays the environmental burden of recycling until the cell is truly spent. After the second life, the battery will be older and perhaps more degraded, but at that point the materials can still be recycled to create new batteries, closing the loop.
Several startups and collaborations (often between automakers and energy companies) are already deploying second-life battery projects. For example, old Nissan Leaf batteries have been used to store energy for buildings and even to power charging stations. As the wave of EVs sold in the last 5-10 years begins to retire, we can expect a huge supply of used battery packs – both a challenge and an opportunity.
Some analyses suggest that for certain chemistries like LFP (lithium iron phosphate), which have long cycle life and contain no high-cost metals, second-life use might be particularly attractive, since the incentive to recycle LFP for its material value is lower (no cobalt/nickel). By giving LFP batteries another decade of use in stationary storage, we defer recycling until there’s larger volume (critical mass) that makes recycling economically viable.
In summary, second-life battery applications can significantly enhance the economics and sustainability of the lithium battery ecosystem. While not every battery will be suitable for reuse, and not all industries can take advantage (for instance, some industrial vehicle batteries are used to near full depletion already), it’s a solution that should be evaluated before recycling. It ultimately reduces waste and maximizes the utility derived from the raw materials (lithium, etc.) that went into the battery.
Conclusions
Lithium-ion battery recycling is entering a new era of innovation and scale-up. The surge in electric vehicles and renewable energy storage is ensuring that enormous volumes of LIBs will reach end-of-life in the coming years – and dealing with this waste stream is crucial for resource sustainability and environmental protection. Current recycling efforts have made progress in recovering valuable metals like cobalt, nickel, and copper, but historically lithium itself has been under-recovered. This is rapidly changing as lithium’s strategic importance grows and technologies improve. Hydrometallurgical processes now can recover lithium at high rates and produce battery-ready compounds, though they must be made more cost-effective. Pyrometallurgical processes, while operationally simple, are giving way to more efficient and greener methods due to their lower recovery and higher emissions.
Looking forward, the industry is moving toward a combination of strategies: advanced recycling technologies (direct recycling, mechanochemical methods, bioleaching) to maximize recovery and purity, second-life battery utilization to extend the service of batteries and defer recycling costs, and design and policy measures to support a circular battery economy. The environmental case is compelling – recycling lithium batteries can dramatically cut CO₂ emissions (up to 60% less than mining and refining new material) and avoid the water use and land disruption of extracting virgin lithium. Economically, while challenges remain in making lithium recovery profitable, the rapid rise in lithium demand and prices in recent years is tilting the equation. Recycled lithium is expected to become a significant portion of supply in the decades ahead, especially in regions with strong regulatory drivers.
To realize this potential, continued R&D and investment are needed. Scaling up new processes from lab to industrial scale, ensuring they are safe and compliant, and integrating them into battery supply chains will be key. Collaboration between battery manufacturers, recyclers, and policymakers can accelerate the development of standards (for battery labeling, collection, transportation) that make recycling more efficient. The concept of batteries as part of a “circular economy” is gaining momentum – where materials are reused and recycled indefinitely, minimizing waste.
In conclusion, lithium-ion battery recycling and reuse technologies have advanced significantly, offering practical pathways to recover lithium and other critical materials at high efficiency. When combined with second-life applications, these technologies can vastly improve the sustainability profile of lithium batteries. As the world leans more on electric power storage, the parallel growth of the recycling industry is not just desirable but necessary. By closing the loop, we can reduce dependency on mining, stabilize material supply, and protect the environment, all while supporting the clean energy transition. The coming years will be pivotal as pilot projects become full-scale operations and the recycling rate of lithium-ion batteries climbs from today’s modest levels toward the goal of near-total recovery.
References
Flash Battery (2021). Recycling processes for battery recovery – Company blog article discussing lithium battery recycling steps, efficiencies, and future process improvements.
Dolotko, O. et al. (2023). Universal and efficient extraction of lithium for lithium-ion battery recycling using mechanochemistry. Communications Chemistry, 6, 49. This study demonstrates an acid-free mechanochemical process achieving ~70% Li recovery from various cathode materials.
Ali, A. et al. (2025). A comprehensive review on the recovery of lithium from lithium-ion batteries and spodumene. Journal of Environmental Management, 391, 126512. An up-to-date review comparing mining vs. recycling of lithium, covering technological, economic, and environmental aspects.