Sections
- The Problem of NO₃⁻ in Feed Waters
- Treatment of Waters with High Nitrate Content
- Concentration of Residues by Vacuum Evaporation
- Conclusions
The Problem of NO₃⁻ in Feed Waters
Water contamination, both surface and groundwater, due to high nitrate concentrations is a widespread and growing problem. This contamination is mainly caused by the massive use of nitrogen fertilizers and poor management of manure in livestock farms.
Consuming water with high nitrate concentrations poses a health risk, especially for children and the elderly, causing a disease characterized by inhibiting oxygen transport in the blood (methemoglobinemia). Additionally, nitrates can form potentially carcinogenic compounds.
Regarding the environment, we face the eutrophication of surface waters, that is, the increase of nutrients in the water (nitrogen and phosphorus) which causes rapid growth of phytoplankton and other aquatic plant species in the waters. Eutrophication can also promote the proliferation of invasive species and significantly increase vegetation.

This growth can be invasive, leading to blooms that can negatively impact water quality through the development of cyanotoxin-producing bacteria.
Suspended matter increases and prevents light from penetrating deep layers, which reduces dissolved oxygen. Bad odors are produced by the emission of methane and hydrogen sulfide. The volume of organic sludge also increases, and anoxia can cause the death of many fish.
There are legislations and limits regarding nitrate concentration, both in drinking water and irrigation water.
Regarding drinking water, European legislation (Directive 91/676/EEC) establishes that the maximum allowed nitrate concentration in water for human consumption is 50 mg/l. However, a lower limit is intended to be set, around 10 mg/l, for greater health safety.
Regarding water intended for irrigation, there are guidelines to avoid water contamination from the application of fertilizers rich in nitrogen and phosphorus. The attached table shows indicative analytical limits for irrigation waters:
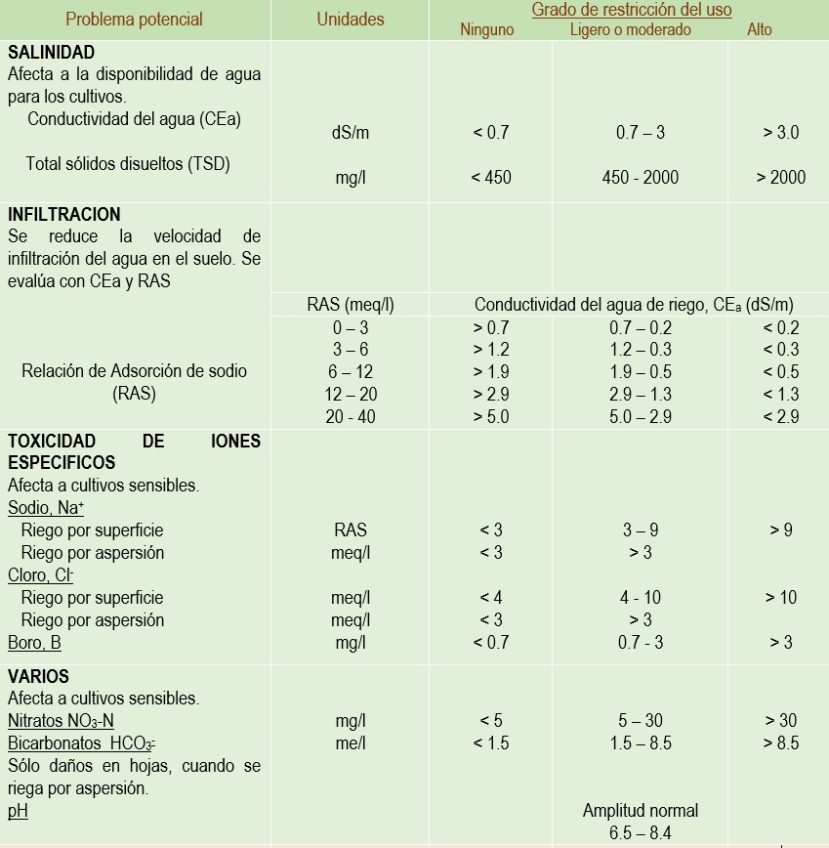
The main parameters to consider when evaluating irrigation water quality include:
- pH, which indicates the acidity or alkalinity of the water.
- Electrical conductivity, which measures the total amount of dissolved salts in the water.
- Nitrate and phosphate levels, as high levels can cause eutrophication.
- Water hardness, determined by the concentration of calcium and magnesium.
- Sodium content, as high content can be harmful to some plants.
- Presence of chemical and biological contaminants such as heavy metals, pesticides, and pathogens.
Treatment of Waters with High Nitrate Content
Nitrate is a stable anion and highly soluble in water. There are physicochemical methods that allow effective removal of nitrates from contaminated waters.
Ion Exchange
Among the available technologies, ion exchange (IX) treatment stands out, which uses columns with anionic resins designed to exchange nitrate ions (NO₃⁻) for anions such as chloride (Cl⁻) or bicarbonate (HCO₃⁻) present in the resin. Once saturated, the resin is regenerated using a concentrated solution of sodium chloride or sodium bicarbonate, generating an effluent highly concentrated in salts.
Ion exchange installations have a compact size, produce high-quality water, and have a relatively affordable operating cost. On the downside, they require a high consumption of regenerants and generate a regeneration effluent containing both the displaced nitrates and the excess regenerant reagent.
The exchange capacity of these resins is relatively low (around 0.5 meq/L), so as nitrate concentration increases, larger columns or more frequent regeneration cycles are required.
For these reasons, ion exchange is considered especially suitable as a polishing process for residual nitrate removal after main treatments, such as separation by reverse osmosis membranes.
Reverse Osmosis
Reverse osmosis (RO) is a very efficient technology for removing nitrates from water. This technology applies pressure to contaminated water to force it through a semipermeable membrane that retains most dissolved solutes, including nitrates. This technology has the following advantages:
- High nitrate removal rate (>90%).
- Does not require addition of chemicals (except those applied in pretreatment, if necessary).
- Produces high-quality water.
Disadvantages include the energy cost derived from pumping water through the membranes and its sensitivity to fouling, which requires pretreatment to avoid premature membrane fouling.
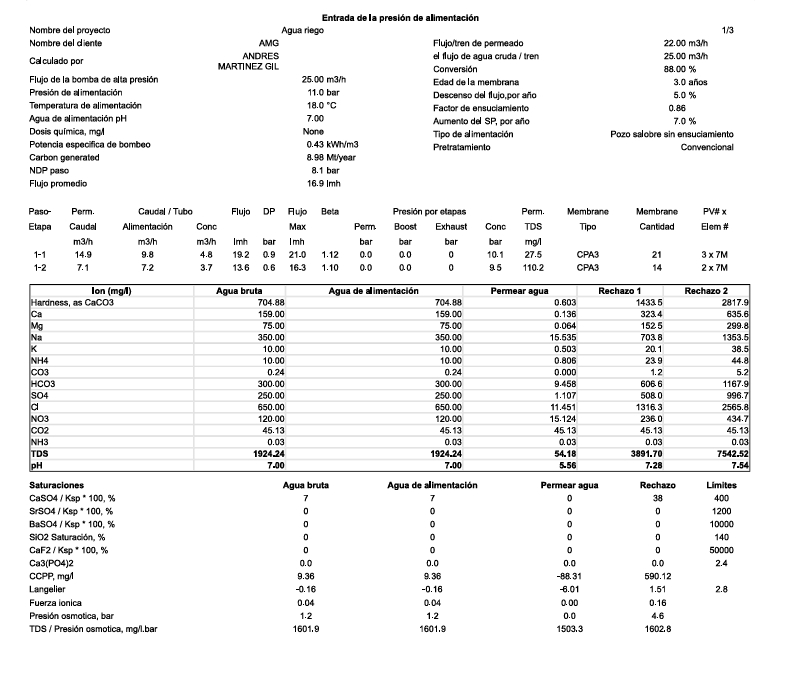
In this context, we have taken as an example an analysis of well water with a high nitrate load and low turbidity. Water treated by reverse osmosis can be used for irrigation or drinking water supply if complemented with appropriate post-treatment.
| Table of Raw and Reverse Osmosis Treated Water Quality | ||||
|---|---|---|---|---|
| Parameter | Unit | Well Water | Irrigation Water – Recommended Limit Values | Osmotized Water |
| Ca⁺⁺ | mg/l | 159 | 400 | 0.15 |
| Mg⁺⁺ | mg/l | 75 | 60 | 0.1 |
| Na⁺ | mg/l | 350 | 900 | 20 |
| K⁺ | mg/l | 10 | 0.6 | |
| HCO₃⁻ | mg/l | 300 | 600 | 10 |
| SO₄⁻² | mg/l | 250 | 1000 | 1.5 |
| Cl⁻ | mg/l | 650 | 1100 | 15 |
| NO₃⁻ | mg/l | 120 | 30 | 15 |
| TDS | mg/l | 1924 | 2000 | 62 |
| Conductivity | μS/cm | 2750 | 3000 | 90 |
| pH | – | 7 | 6.5 – 8.5 | 5.6 |
| Turbidity | NTU | ≤ 10 | ≤0.1 | |
| SS | mg/l | 20 | ≤0.1 | |
| OM | mg/l | ≤10 | ≤1 | |
| Oils and Fats | mg/l | ≤2 | ≤0.1 | |
There are other highly promising treatments for nitrate removal that do not generate residual streams as physicochemical treatments do, highlighting biological denitrification and catalytic denitrification, currently under development.
Concentration of Residues by Vacuum Evaporation
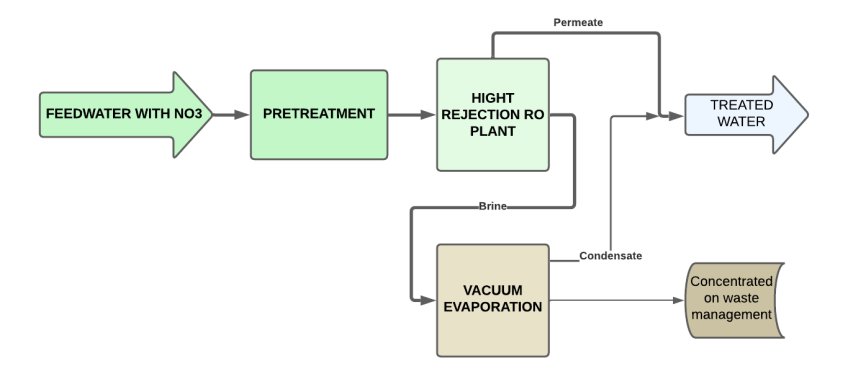
Reverse osmosis is a good solution for treating waters with high nitrate concentrations, but it produces a contaminant concentrate known as RO reject, which must be properly managed due to its high contaminant concentration.
An effective solution to treat the brine resulting from reverse osmosis is vacuum evaporation, a thermal technology that allows concentrating liquid residues until reduced to a minimal volume. The process consists of:
- Reducing system pressure to lower the water boiling point (usually below 50 °C).
- Applying heat to evaporate the water, which then condenses as distillate.
- Concentrating residues into a smaller liquid fraction or even solid, which can be managed as controlled industrial waste.
This system allows recovering up to 95% of the water present in the reverse osmosis reject and significantly reduces the volume of residues.
Vacuum evaporation allows treatment of complex mixtures, which is unfeasible by conventional techniques, obtaining quality water and a highly concentrated residue that can be easily managed.
The range of vacuum evaporators from Condorchem Envitech includes the main types of these units:
- Low-temperature vacuum evaporators by heat pump
- High-temperature vacuum evaporators by mechanical vapor compression
- Multiple-effect vacuum evaporators
- Crystallizers
Crystallizers are usually applied as the final phase of treatment to completely dry the brine residue.
In some cases, the most efficient solution is the combination of both technologies, especially when membrane filtration technologies are not sufficient to achieve the desired results and it is necessary to extend treatment by evaporation and/or crystallization.
Advantages of the Combined Process
The integration of reverse osmosis with vacuum evaporation offers a robust and sustainable solution for treating waters with high nitrate concentration. Its main advantages are:
- Production of safe drinking water.
- Minimization of waste volume.
- Possibility of reusing treated water for other purposes (irrigation, industry, etc.).
- Compliance with discharge regulations and reduction of environmental impact.
Forced circulation vacuum evaporators are commonly used to concentrate saline rejects with high nitrate content coming from a reverse osmosis system.
If the goal is to optimize energy consumption, the following types of evaporators can be used:
- Multiple-effect evaporator, which uses the vapor from the first effect to heat the following ones, reducing energy consumption.
- Mechanical vapor compression (MVC) evaporators, which reuse compressed vapor as a heat source and are ideal for treating medium volumes with high energy efficiency.
The evaporator concentrate (also called concentrated residue or residual brine) contains nitrates and other salts that could not be removed in previous stages. The final destination of this residue depends on several factors:
- Volume of residue.
- Local regulations.
- Type of contaminants.
- Available budget.
The most common options are:
- Storing the concentrate in tanks or containers and delivering it to an authorized hazardous or special waste manager, in case certain toxicity levels are exceeded (for example, high nitrate or heavy metal levels). This is a safe and regulated option, although it has a high cost per ton, so it is a priority to increase sludge concentration as much as possible.
- If the evaporator is followed by a crystallization stage, salts can be recovered in solid form. These salts, if inert or valorized, could be destined for secondary uses (such as fertilizers) or sent to a controlled landfill.
Conclusion
In conclusion, nitrates are highly soluble salts and difficult to separate from water, both in natural sources and wastewater. In the case of water intended for human consumption, reducing them to acceptable levels is essential to protect health, given the demonstrated harmful effects. Likewise, ensuring the quality of water used for irrigation is essential, as it directly influences plant health, crop yield, and consequently, the agricultural economy.
Regarding discharges, it is essential to comply with regulations on maximum nitrate limits to avoid eutrophication phenomena. This can lead to biological overpopulation processes and massive blooms, with negative consequences for the balance of the aquatic ecosystem.
The most commonly used solutions today include filtration by reverse osmosis membranes, complemented, if necessary, with specific ion exchange resins to remove residual nitrates.
The residues generated in these processes have a high concentration of contaminants, including nitrates themselves, so they require proper treatment before disposal in authorized landfills. In this regard, vacuum evaporation and/or crystallization stand out as effective technologies to minimize the volume of these residues.
Finally, it is worth remembering that the best treatment is always prevention. Responsible application of fertilizers on agricultural soils and proper control of urban and industrial wastewater are fundamental to avoid nitrate contamination from the source.
Bibliography and References:
Hydranautics – A Nitto Group Company, IMSDesign
Water Quality Table for Irrigation: Essential Guide to Optimize Your Crop Health | Water Institute
Water Desalination by Vacuum Evaporation Systems | Condorchem Enviro Solutions
Water Quality for Irrigation: Complete Guide to Ensure Healthy and Sustainable Crops | Water Institute
Current Treatments in Nitrate Removal – El Agua