Sections
- The Selective Catalytic Reduction (SCR) Process
- SCR Applied to Wastewater Treatment
- Description of the SCR Process Applied to Effluent Treatment
- Conclusions
The Selective Catalytic Reduction (SCR) Process
The emission of nitrogen oxides is very dangerous to health, as it affects the respiratory systems of people and animals, potentially causing respiratory and cardiovascular diseases due to its acidic nature. Furthermore, once emitted, they can give rise to other secondary pollutants. The reactions produced in the atmosphere by these compounds are very complex and involve radicals such as OH and O₃.
Nitrogen oxides (NOx) are present in the exhaust gases of boilers, diesel engines, power generation plants, and industrial processes, among which the following deserve special mention:
- Energy Industry: Thermoelectric and cogeneration plants.
- Diesel Engines: Freight vehicles, heavy machinery, and ships.
- Cement and Metallurgy: Emissions from furnaces and boilers.
- Chemical and Petrochemical: NOₓ formation in refineries and combustion processes.
These compounds significantly contribute to air pollution and cause the following harmful effects:
- Destruction of atmospheric O₃
- Contribution to the greenhouse effect
- Production of acid rain
- Traffic pollution (Smog)
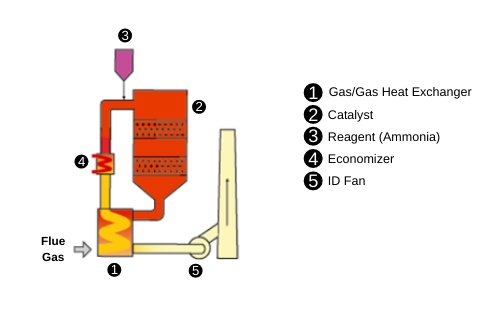
Selective Catalytic Reduction (SCR) is a process used to convert nitrogen oxides (also known as NOx) into diatomic nitrogen (N₂) and water. This is achieved with the help of a reducing agent, usually ammonia (NH₃), (NH₄OH), or urea (CO(NH₂)₂), which is added to the contaminated flue or exhaust gas stream and reacted with a catalyst. When using urea, nitrogen (N₂) and carbon dioxide (CO₂) are produced.
An SCR system basically consists of:
- Reducing agent: Ammonia, in the form of NH₄OH, or urea is vaporized and diluted with air to be injected directly into the gas stream to be treated through a distributor.
- Catalytic reactor: This is the chamber where the reaction of NOₓ with the catalyst takes place. The catalyst is composed of titanium salts (TiO₂), tungsten (WO₃), or vanadium (V₂O₅). Zeolites impregnated with copper or iron can also be used.
Operating Principle
Selective catalytic reduction uses a reducing agent, generally ammonia (NH₃) or a urea solution (NH₂CONH₂), which reacts with nitrogen oxides in the presence of a catalyst. The main chemical reaction is:
4NO + 4NH₃ + O₂ → 4N₂ + 6H₂O
The following reaction can also occur:
6NO₂ + 8NH₃ → 7N₂ + 12H₂O
The process is highly efficient, achieving up to 90% reduction of NOₓ present in gaseous effluents.
SCR Applied to Wastewater Treatment
Selective catalytic reduction (SCR) is widely used in the treatment of nitrogen oxide emissions and, although less common, there is also a technology based on selective catalysis in wastewater treatment, which is used for the degradation of nitrogenous and refractory organic compounds.
In the case of wastewater containing high concentrations of NOx, the selective catalytic reduction (SCR) process is designed to reduce these compounds by injecting ammonia (NH₃) in the presence of excess oxygen (O₂) and a suitable catalyst. As a result, NOx is transformed into harmless compounds such as nitrogen (N₂) and water vapor (H₂O).
The removal of NO₃⁻ and NO₂⁻ (denitrification) is necessary in industrial and municipal wastewater containing excess nitrates and nitrites, which can cause eutrophication in the discharge. For this, heterogeneous catalytic reduction is used, in which a catalyst (generally based on metals such as platinum, palladium, or copper) facilitates the reduction of nitrates to molecular nitrogen (N₂), avoiding the formation of ammonia (NH₃), which is another pollutant to be avoided in the discharge.
- Main chemical reaction: NO₃⁻ + 2H₂ → N₂ + 2H₂O
Hydrogen (H₂) sources or reducing agents such as formic acid or acetic acid can also be used.
Advantages
- High efficiency in the removal of nitrates and nitrites.
- Does not generate hazardous secondary waste.
- Fast and stable process compared to biological treatments.
- Can be applied in combination with other treatments, such as filtration and adsorption.
Disadvantages
- Requires specific catalysts, which can be costly.
- Dependence on controlled pH and temperature conditions.
- Cost of a reducing agent (H₂, acids, or peroxides).
- Possible catalyst deactivation over time due to fouling or contamination.
Removal of Persistent Organic Pollutants (POPs) and Emerging Contaminants
Some effluents contain compounds that are difficult to degrade with conventional biological procedures, such as those from the pharmaceutical industry, pesticides, phenols, and dyes. Selective catalytic oxidation (similar to the SCR process) uses metallic catalysts and hydrogen peroxide (H₂O₂) or ozone (O₃) to break them down.
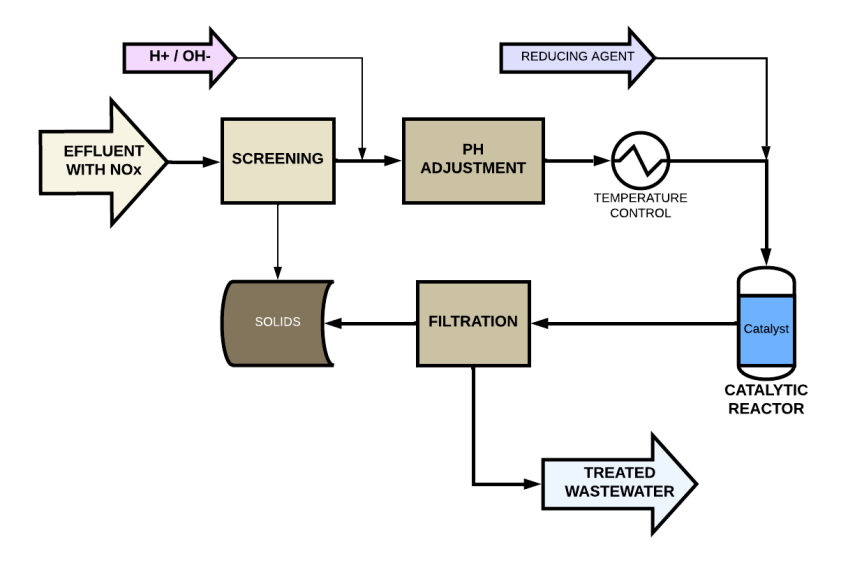
Description of the SCR Process Applied to Effluent Treatment
An effluent treatment process using selective catalytic reduction consists of the following stages:
Effluent Pretreatment
Consisting of:
- Preliminary solids separation by screening or filtration.
- pH adjustment (6.5 – 8.5) with alkali or acid.
- Temperature control (10 – 50 ºC) using a heat exchanger, if necessary.
Injection of the Reducing Agent
The following are used:
- Hydrogen (H₂) for metallic catalysts such as platinum or palladium.
- Formic acid (HCOOH) or acetic acid (CH₃COOH), used with copper or silver catalysts.
- Carbohydrates or ethanol, used in processes with biocatalysts.
Catalytic Reactor
Inside the catalytic reactor, nitrates (NO₃⁻) and nitrites (NO₂⁻) react with the reducing agent in the presence of a catalyst, whose function is to accelerate the conversion of nitrogen compounds into molecular nitrogen (N₂), which is released into the atmosphere.
Common catalyst materials:
- Noble metals: Platinum (Pt), palladium (Pd), rhodium (Rh).
- Transition metals: Copper (Cu), iron (Fe), nickel (Ni), silver (Ag).
- Ceramic supports: Titanium oxide (TiO₂), alumina (Al₂O₃), zeolites.
Main reactions:
NO₃⁻ + 2H₂ → N₂ + 2H₂O
NO₂⁻ + H₂ → N₂ + H₂O
Product Separation and Final Filtration
After catalytic treatment, colloidal products or precipitates may be generated, which can be removed by the following processes:
- Sand or activated carbon filters
- Centrifuges or sedimentation tanks
Effluent Treatment with NOx and SO₂
Wastewater containing nitrates (NO₃) and sulfur dioxide (SO₂) can be treated by selective catalytic reduction. This technology offers excellent results when reducing concentrations of NO₃ and SO₂, two common pollutants in wastewater from the agricultural sector and various industrial sectors.
The treatment line is similar to that described for NOx treatment, but the presence of SO₂ must be taken into account, as compounds such as H₂SO₄ and (NH₄)₂SO₄ may form. These compounds can be problematic for installations due to their high corrosiveness and for the catalytic process, as they can clog the catalyst bed.
Reduction of Sulfur Dioxide (SO₂) to Sulfur (S):
During the SO₂ reduction process, sulfur dioxide is transformed into elemental sulfur or other less hazardous sulfur compounds.
- The typical catalytic reduction process converts SO₂ to elemental sulfur.
- The catalyst helps facilitate the reduction, and base metals such as nickel or copper can be used.
- Temperature and reactant concentration must be controlled to avoid the formation of undesired compounds, such as sulfuric acid (H₂SO₄).
Advantages
- High efficiency. It is an efficient process with a purification performance >90%.
- Selective and controlled reactions. It is feasible to reduce NO₃ and SO₂ concentrations without significantly affecting other compounds present in the wastewater.
- Recovery of valuable products: The sulfur obtained can be recovered and used in other industrial processes.

Disadvantages
* Operating costs: Operation and maintenance costs can be high due to the use of specific catalysts and reagents.
* Catalyst replacement: Over time, catalysts lose their effectiveness and may require regeneration or replacement.
* Process control: Strict control of temperature, pH, and reactant concentration is required to achieve the expected SCR efficiency.
Conclusion
The presence of NOx in gaseous emissions and discharges must be treated to eliminate the risk they pose to health and to comply with legal limits.
A large part of NOx emissions into the atmosphere come from industrial processes and energy production.
Selective Catalytic Reduction (SCR) is an efficient method to reduce nitrogen oxides (NOx) in industrial emissions by using a catalyst and a reducing agent such as urea or NH₃. With this treatment, NOx is transformed into nitrogen and water vapor, minimizing its environmental impact.
Although this technology is better known for treating gaseous emissions, it is also widely used for reducing NOx and recalcitrant organic compounds present in wastewater. This system integrates a set of advanced technologies including catalytic reactors, dosing systems, mixing and filtration equipment, as well as monitoring sensors. Proper integration allows efficient removal of nitrogenous and organic pollutants.
In the case of nitrate (NO₃⁻) and nitrite (NO₂⁻) reduction, SCR converts nitrogen compounds into molecular nitrogen (N₂), preventing their accumulation in water and avoiding environmental problems such as eutrophication.
In the presence of SO₂, care must be taken in process design to prevent clogging and deterioration of the installation due to possible formation of H₂SO₄ or (NH₄)₂SO₄. Appropriate catalysts and strict control of operating conditions must be provided.
Bibliography and References
Selective Catalytic Reduction (SCR) | Condorchem Enviro Solutions
Selective Catalytic Reduction _ AcademiaLab
NOx | Selective Catalytic Reduction (SCR) | NOx Emission Treatment | Condorchem Enviro Solutions